July 6, 2022
BIOINVEST NEWS: Precigen (PGEN)
Sale of Non-Core Assets For $170 Million Will Pay Off Convertible Debt And Remove Overhang – BUY
Precigen announced the sale of a wholly-owned non-healthcare subsidiary, Trans Ova Genetics, L.C. (“Trans Ova”) for $170 million. The proceeds will go towards retiring roughly $200 million in convertible debt due in 2023. With several other non-core assets available for divestiture plus the increasing likelihood of a value added corporate collaboration this year, Precigen is one of the rare early-stage biotechs with debt that has now proven the ability to pay off it’s debt on time without the need for dilutive financing or impacting its broad and deep health care clinical focus. A major financial overhang has been removed. We reiterate our STRONG BUY and recommend subscribers take advantage of the very attractive entry point in the stock as management has proven its ability time and again to deliver on stated clinical and now financial objectives. Several more catalysts ahead. BUY
One Down, More To Come
The sale of Trans Ova, an industry-leading animal reproductive technologies company, to URUS, a global agriculture holding company will close this quarter and bring in $170 million in cash, plus a $10 million earned out over two years. Moreover, the Company owns three other non-core business that we believe will lead to at least one more sale soon. For example, Precigen Exemplar (genetically engineered swine models) is another division that no one talks about and possibly could bring in roughly $50 million in a sale. We estimate the Company ended Q2:22 with ~$125 million in cash and is spending roughly $15+ million per quarter; hence about two years in cash without any additional deals – but more are coming (without the need for dilute financing and after more value added clinical data is due).
Deep Pipeline Offers Various Deal Potential
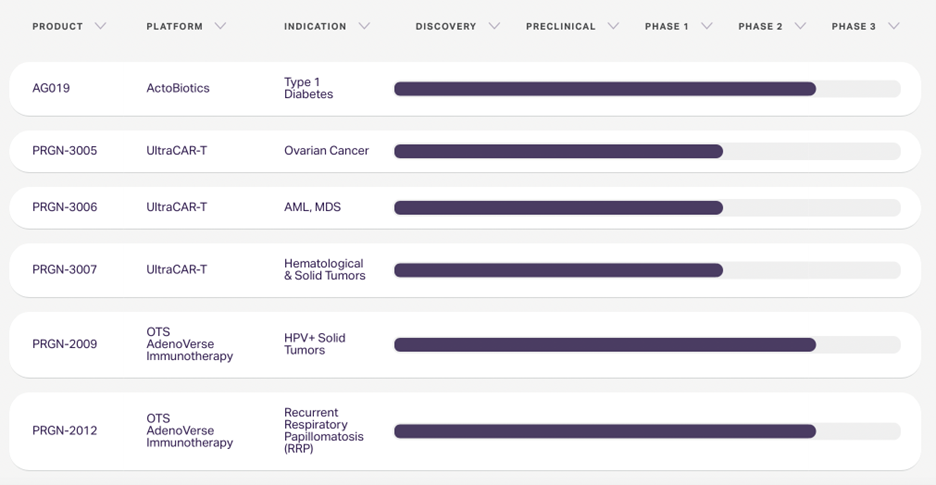
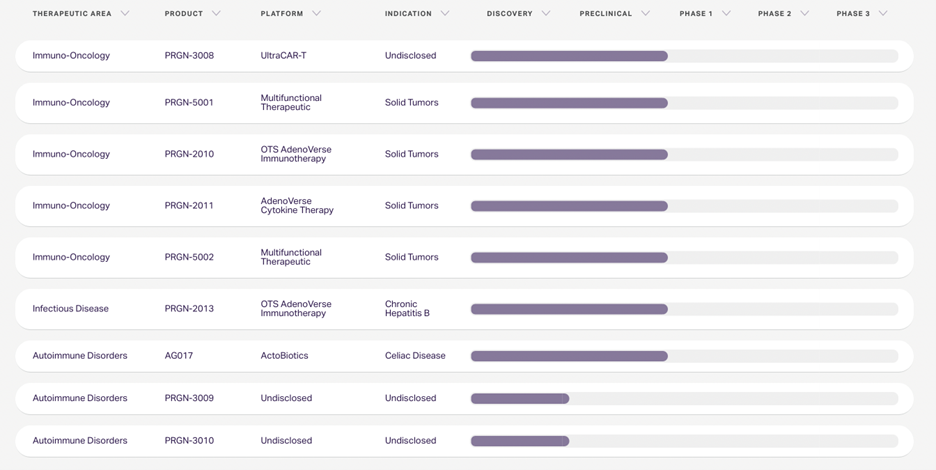
For a while we have believe that PGEN would, should and could form a corporate partnership for UltraCART and/or AdenoVerse platforms. The charts below show both the Clinical and Pre-Clinical Portfolios at Precigen. Clearly, management has been focused on addressing the debt situation – and the TranOva sale removes the debt overhang. Now we believe we will see additional strategic joint ventures that will bring in both quality partners and the resources to continue or accelerate development of the pipeline.
Clinical Pipeline (as of July 5, 2022)
Pre-Clinical Pipeline (as of July 5, 2022)
Below is a Summary of Compound Precigen Updates and Catalysts Since Our January Update (in Bold)
PRGN-3006 UltraCAR-T – Initiation of an expansion phase of the study at Dose Level 3 with lymphodepletion in happening now. In the first cohorts in AML, objectives responses of 33% (1/3) and 66% (2/3) were seen with clean safety (no DLTs or neurotox). Additional Phase 1/1b data is expected in 2022. The FDA granted ‘3006 Fast Track Designation in April.
PRGN-3005 UltraCAR-T is in a Phase 1/1b trial to treat patients with advanced, recurrent platinum-resistant ovarian cancer. Excellent CAR-T expansion and duration was seen with a single dose. The company has received FDA clearance to incorporate lymphodepletion and has now begun Dose Level 3 of the IV arm and will initiate the expansion phase of the study, including redosing.
PRGN-3007 program: A Phase 1 study in receptor tyrosine kinase-like orphan receptor 1 (ROR1) plus hematological (chronic lymphocytic leukemia, mantle cell leukemia, lymphoblastic leukemia, diffuse large B-cell lymphoma) and solid (triple negative breast cancer) tumors will begin in 2022. ‘3007 has an intrinsic down regulation of PD-1 on Ultra-CAR-T cells to avoid systemic PD-1 blockade.
PRGN-2012 is in a Phase 1 trial as an adjuvant immunotherapy following standard-of-care surgical removal of visible papillomas in adult patients with Respiratory Papillomatosis (RRP). A Phase 2 trial has just begun. Additional Phase 1 expansion data is expected in the H2:22. See the impressive slide above.
PRGN-2009 is a Phase 1 trial as a monotherapy (Arm A) and in combination with M7824 (Arm B) in previously-treated patients with recurrent or metastatic HPV-associated cancers. A Phase 2 study in advanced HPV-associated cancer indications in combination with an approved anti-PD-1 checkpoint inhibitor has begun. HPV cancers remain a significant market with poor treatment options. See slide above.
The company plans to seek FDA guidance on a rapid regulatory strategy for PRGN-2012 and PRGN-2009.
AG019 ActoBiotics: PGEN also plans to begin discussions with the FDA and European Medicines Agency on the Phase II/III clinical trial design for their oral treatment, AG019 in Type 1 diabetes.
Still One of Our Favorites
Now that the financial overhang is mostly behind us and the pipeline progress continues ahead unabated, we believe PGEN is very attractive at current levels. The Company is also expected to hold an investor R&D Day in the coming months to show the progress in at least three major compounds and maybe others in the pipeline. We urge subscribers to take advantage of a small cap biotech company stock with one of the most unique and broad platforms around and just as importantly, with management that is delivering on all fronts.