MTSL Issue 982
IN THIS ISSUE: Lots Of Good Biotech News These Days; The Medicare Terms In Biden Inflation Reduction Act – Good For Biotech?
Since Last Issue: BTK: 4.52%; NBI: 6.22%; XBI: 10.63%; Model Portfolio: 11.40%
SENTIMENT — Overbought? Seriously? Yes And We’ll Take It
COVID News: Summer COVID Infections Peak; Sales for MRNA and PFE/BNTX Beat High Expectations While NVAX Flunks Again
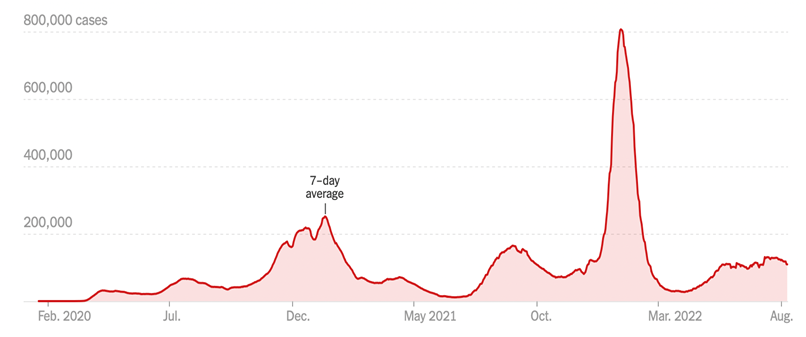
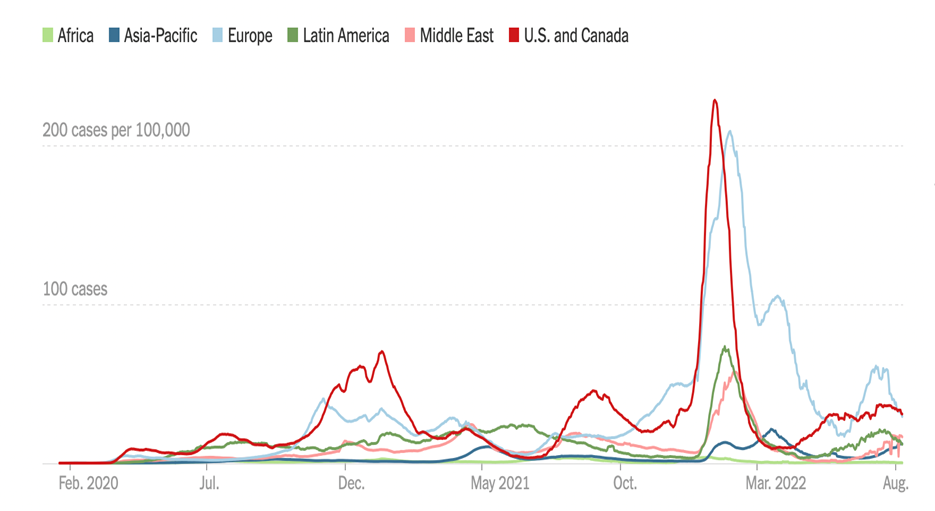
COVID – Has This Wave Peaked?
Positive cases are down a little since the last Issue, at ~110,000 a day (versus -15% over the past two weeks; it was -2% last Issue). Most regions are declining (except select states Alaska, Iowa, Pennsylvania). Deaths, a lagging indicator, are up 13% (vs. 10% during the same period two weeks earlier. Omicron B.5 remains the dominant strain, and for the most part symptoms are mild (e.g, President Biden), especially if you have been vaccinated and then boosted. Flights are packed, hotels are full – for the vast majority, life is back to normal.
ROW Down Too
The rest of the world COVID cases are also down around the same pace (-15%) as U.S, with European cases falling nicely as well as in Asia/Pacific and Latin America, too, since the last Issue.
Has Vaccine Demand Peaked? MRNA Flying While NVAX Flounders Again
Moderna is now firmly established as the COVID vaccine leader, with $4.3 billion in Q2 sales and about $21 billion for 2022 (on 8/3). Another 15 million doses were just purchased by the EU (on 8/10), although demand appears to have peaked. When Novavax announced Q2 results and a 2022 outlook that was much lower than just last quarter’s outlook, it appears to us that COVID vaccine demand has peaked. NVAX forecasts 2021 COVID vaccine revenue of $2.0-$2.3 billion, way less than the $4.5-$5 billion predicted three months ago (talk about poor execution all along). Governments are looking for newer bivalent booster vaccines that both MRNA and PFE/BNTX are well into developing. Boosters are the way to go for now, and predominantly targeting the Ba.4 and Ba.5 strains.
SENTIMENT – So Much Good News & Yes We Hit Overbought Levels
Biotech experienced a bunch of really good fundamental events of late. First, positive late-stage data is a major driver of biotech stocks. On August 4, investors got the Big One when Alnylam (ALNY) delivered stellar data for its Patisiran compound in the APOLLO-B trial of ATTR amyloidosis with cardiomyopathy. Other ATTR stocks rallied (MTSL’s IONS). The data set the group on fire and it has not turned back since. ALNY shares exploded up 50% on the day, a market cap increase of ~$8 billion. There’s been several additional big Phase III wins too (e.g., KRTX, etc.)
Next, biotech takeovers at huge premiums have occurred (and the widely rumored SGEN/MRK takeover hasn’t been announced). AMGN is buying ChemoCentrix for $3.7 billion – a premium of 115% to the target’s previous closing price. CCXI’s lead product is Tavneos, a selective complement component 5a receptor inhibitor that was approved by the FDA in October 2021 for adult patients with severe active ANCA-associated vasculitis. Other inflammation/autoimmune stocks have also rallied in sympathy. Then Pfizer announced it is acquiring Global Blood Therapeutics (GBT) and its sickle cell anemia drug Oxbryta for $5.4 billion, at another premium of almost 100% when the news began to circulate. Stocks in the sickle cell field also have risen (e.g., CRSP, SGMO). While not a takeover, small cap Mersana (MRSN) formed a solid early stage collaboration with GSK for its antibody-drug conjugate (ADC), XMT-2056, The global deal gives GSK an exclusive option to co-develop and commercialize XMT-2056. GSK will pay $100 million upfront, plus Mersana could make up to $1.36 billion in biobucks, making the deal one of the largest ever for a preclinical ADC asset. MRSN is up around 50% since the deal was announced, and the news is another reason smaller, earlier-stage biotech stocks are doing well, too.
BMS is partnering with private biotech GentiBio a biotech firm that is developing regulatory T cells, otherwise known as Tregs, a specialized type of T cell that can modulate both the innate and adaptive immune systems and suppress immune responses that can lead to inflammation that plays a significant role in IBD. The partnership’s financial terms include an undisclosed cash payment made to GentiBio. As the program unfolds, GentiBio stands to make up to $1.9 billion in biobucks, including development and commercial milestone payments, as well as royalties on any approved therapeutics. The goal with Tregs is to develop potential functional cures. MTSL Rec SGMO is a leader in Treg development and is just about to begin trials with its lead Treg compound in renal transplant rejection.
FDA approvals are yet another important driver of biotech stock performance. Since the last Issue, MTSL Recommendation MYOV received an important on-time regulatory OK for Myfembree in endometriosis (see MYOV below). This on top of MTSL Rec INCY’s two recent OK’s for topical ruloxitinib. The FDA approved tirzepatide LLY’s (Mounjaro) for the treatment of Type 2 diabetes in adults. Tirzepatide’s FDA approval marks the first in a new class of diabetes medications: a dual GIP/GLP-1 receptor agonist.
Last but definitely not least, sales and earnings count a lot for later-stage biotech stocks. During this quarter, we saw some very positive quarterly earnings results from both mega-, large- and middle caps (SRPT, VRTX, REGN, Recs BMRN and MYOV, etc.)
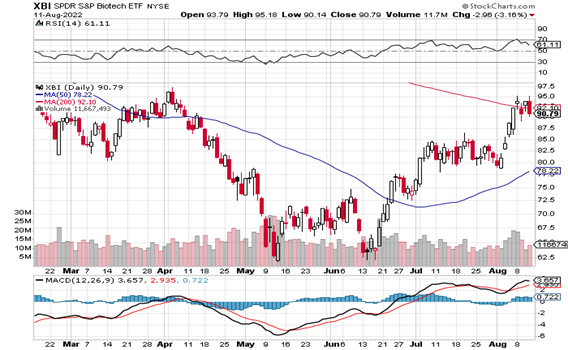
The summer biotech rally continues – and while not over-exuberant compared with 2020/early 2021, by the size of the individual stock moves on great news (many 50%-100%), this past week the XBI did touch overbought conditions. Some cash is being to put to work – as the two recent takeovers alone will return over $10 billion to investors. Last Issue we said that as the group is still overall under-owned and undervalued, then it wouldn’t take much to move the needle. Well, take a look at the XBI charts – the needle has been moved in the longs’ favor. We wouldn’t be surprised if some short-term profit taking occurs, but the group is back in favor for sure.
30 Year Treasury Yield Vs. XBI
T-Bill vs XBI Gap Shrinks Further
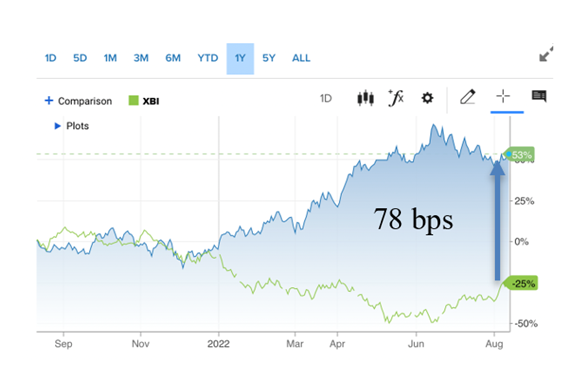
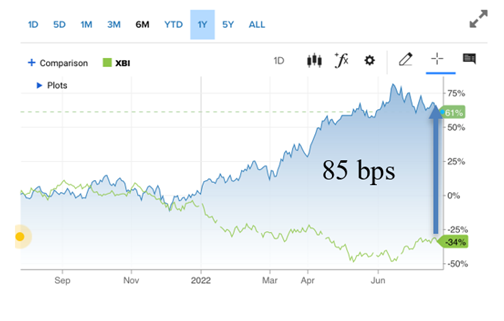
This week’s economic data was the first bonafide signal that inflation has peaked and that the Fed’s strategy is beginning to work. Yes, it is resulting in lower corporate earnings and higher unemployment. Above is our chart comparing the riskier biotech index, the XBI, with the 30-year Treasury bond. We’ve used this measurement to show the fear and also risk appetite of investors over time, in particular as rates are being raised dramatically to reign in runaway hyperinflation. The gap between the two indices is still wide, but keeps narrowing (78 basis points this Issue versus 85 basis points last Issue and 94 the Issue before). It also tells us how well the bios have done over the same period.
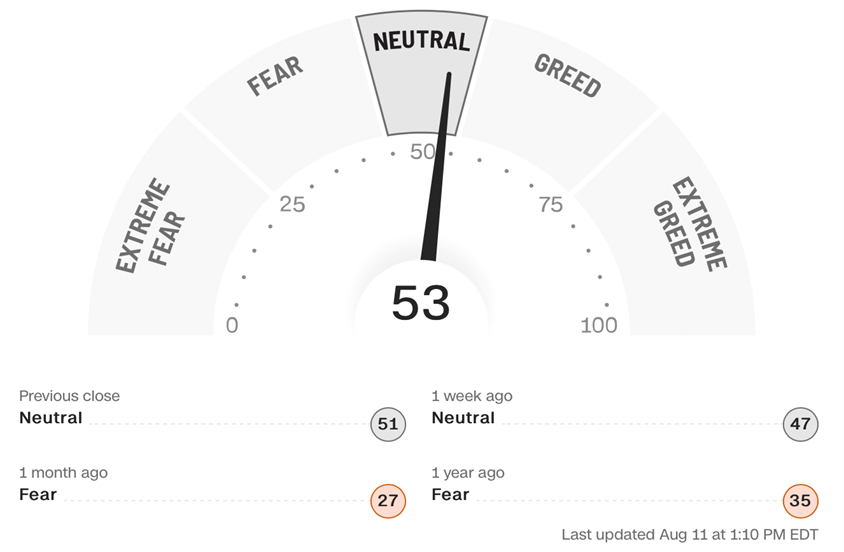
F&G Index Out of FEAR
The Fear & Greed index is now in the NEUTRAL zone (53), up from FEAR last Issue (and EXTREME FEAR two Issues ago). All the hard assets are falling, (real estate, gas and oil prices, and commodities) and continue to decline. Ukraine war is still keeping inflation high but it really seems to have peaked (i.e., not going any higher). Disney’s recent earnings revealed that park traffic has been solid (while streaming services are dropping). The market’s rally is a result of the Fed actions being somewhat successful at this point, and investors now believe that rate hikes and inflation may subside soon – hence the improvement in the F&G Index and the NASDAQ overall. And bios, too. However, we do want to remind investors that neutral is neutral – the market is out of the fear zone, so we may see some profit taking in the days/weeks ahead.
TECHNICALS – Good News All Around, The XBI Touched Overbought
Do you realize that the XBI has rallied almost 50% from the lows in June? The XBI closed at 91 basically up from 82 in the last Issue. Not only well above the rising 50-day MA (78 up from 75), but the Index actually closed right at/ just below the 200-day MA (92) – for the first time in ages. The RSI is now 61 (up from 56 last time), and did in fact touch overbought (70) briefly on Monday (8/8). The MACD is also solidly positive (but maybe plateauing) as investors have allowed the summer rally to continue. Another reminder, summer rallies often peak well before Labor Day as funds try to jump ahead of others who have prepared for a summer rally (LOL). All the good news is being rewarded – but in some cases in the short-term it does appear overly enthusiastic. We would not be surprised to see some short term pullback in prices. So far, the XBI based well before touching the 200-day support level. Should it continue the sideways action again, it may set the stage for another leg up as the year continues. (Or the summer rally can just fade…..)
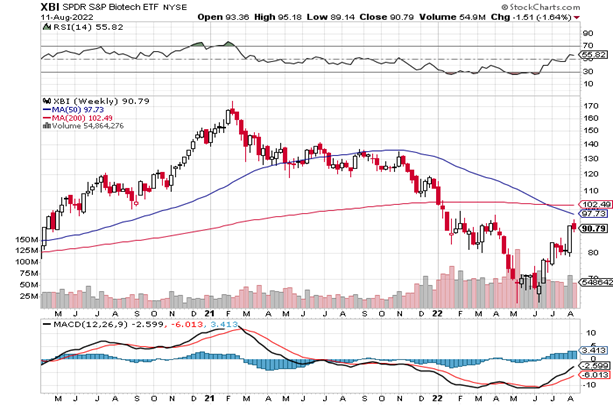
The long-term weekly XBI has been strong since the last Issue and the RSI at 55 – is also up from 47. The Index is slightly below the 50–week moving average (still around 98) and is still below the 200-week (102-103). The 50-week MA had been moving down, but with the strong action in the XBI of late we expect that to level off soon (and maybe then turn up?). As we said last Issue, with a bunch of solid fundamental events clustered in a short time frame, the sector is acting better than it has since the good old days of 2020. Look for more good (and bad) news as the busy post-Labor Day season for health care begins.
Medicare Provisions of the Inflation Reduction Act of 2022
Maybe Not As Bad As Believed; Could Be A Boon For Innovation & Therefore Biotech — Also last Issue, we said that the recently agreed upon reconciliation bill that included the Black Swan scenario of negotiated Medicare drug prices for the first time ever, ironically might be a boon for the biotechs. We predicted that cash hoards at Big Pharma/Bio are being put to work and we have seen more M&A activity recently (e.g., AMGN/CCXI, PFE/GBT). Below are the terms of the current bill as it heads into law.
Prescription Drug Prices Goes into Effect in 2026
The Act enables Medicare to negotiate certain high-cost prescription drug prices covered by Parts B or D. The current law, which was enacted as part of the 2003 Medicare Modernization Act establishing Part D, prohibits price negotiations under Medicare. HHS is authorized to negotiate the prices of 10 prescription drugs in 2026, and another 15 drugs in 2027 and 2028. The number rises to 20 drugs a year in 2029 and beyond. This provision applies only to prescription drugs that have been on the market for several years without competition.
It also includes a provision penalizing drug companies that increase prices for Medicare-covered prescription drugs in excess of the rate of inflation.
Out-of-Pocket Drug Costs Capped At $2000
The Act limits out-of-pocket costs for prescription drugs for Medicare Part D beneficiaries to $2,000 in any one year, including an option to break that annual amount into monthly payments. Currently Part D’s catastrophic cost limits apply after beneficiaries spend $7,050 out-of-pocket. Beneficiaries then pay 5% of subsequent drug costs without limitation. Under the Act’s provision, once a Medicare beneficiary’s annual out-of-pocket expenses for covered drugs reach $2,000, co-insurance costs will be eliminated, as Medicare Part D will pay 20% of the cost of brand-name drugs and 40% of the cost of generic drugs. Insurers and drug manufacturers will bear the remaining costs. Capping out-of-pocket drug spending under Medicare Part D would especially help those who take high-priced drugs for conditions such as cancer or multiple sclerosis.
In addition to this annual cap, the Act creates a monthly cap of $35 on insulin costs under Part D. An estimated 3.3 million Medicare beneficiaries use some forms of insulin. It also includes a provision to further limit out-of-pocket drug costs for lower-income Medicare Part D beneficiaries and people with disabilities. This provision expands eligibility for Part D low-income subsidies to beneficiaries with incomes of up to 150 percent of the federal poverty level.
Vaccines Included
The Act makes certain vaccines free for Medicare Part D beneficiaries by eliminating any cost-sharing for such vaccines. According to KFF, 4.1 million Medicare beneficiaries received a vaccine covered by Part D in 2020, including 3.6 million who received shingles vaccines.
Implications For The Biotech Sector Are Positive
We believe that this deal, which might be a long-term negative for Big Pharma/Biotech, is good for smaller biotech companies as innovation and patented, single-sourced drugs could remain free of government price negotiations for at least the foreseeable future. The deal may be the beginning of what some consider government price controls, so we still need to watch as developments progress.
Clinical Trials Watch
Company Updates
ACAD — Reports Strong Q2 Revenue, Trofinetide NDA Submitted, Stops Nuplazid in Alzheimer’s Disease Psychosis, Drops Two Programs; ACP-044 & ACP-319
ACAD held a busy quarter call
The Company announced that following the recent CRL from the FDA regarding Nuplazid in Alzheimer’s disease psychosis (ADP), they have decided to not run another study in ADP and will no longer be developing Nuplazid for ADP or any other dementia-related psychosis (DRP) indications. As the CRL was widely expected, the stock reacted positively to the news as uncertainty regarding the ADP opportunity was understandably a major overhang on the stock. With the ADP uncertainty removed and the program re-priortization (dropped ACP-044 & ACP-319), investors are realizing that Acadia has a deeper R&D pipeline while Nuplazid continued to deliver solid sales.
Nuplazid Still Solid
Nuplazid’s Parkinson’s disease psychosis (PDP) revenue was $135 million (+17% QoQ) vs. the consensus estimate of $130 million despite the company noting continued COVID-19 headwinds in the Parkinson’s disease market as patient office visits and long term care (LTC) occupancy rates remain depressed. The Company lowered the top-end of FY22 guidance to $510-540 million from the previous guidance of $510-560 million.
Trofinetide NDA Next
ACAD recently submitted an NDA for trofinetide for the treatment of Rett syndrome in adults and pediatric patients two years of age and older. ACAD expects a Priority Review with an action date, most likely in Q1:23. The compound resulted in positive topline results from the Phase III (LAVENDER) trial evaluating IGF-1 analogue in females aged 5-20 years old with Rett syndrome (n=187).
There was a high rate of treatment emergent adverse events (TEAEs), specifically diarrhea, which led to a 17% discontinuation rate in the treatment arm vs. 2% in placebo and was a potential problem. ACAD is currently implementing a diarrhea management plan in the open label extension studies and management noted on the call that they are seeing positive results so far. In our view, the Phase III data were encouraging and build on the earlier positive Phase II data and have the potential to lead to an FDA approval.
Drops Two Other Programs
The Company also announced that they have decided to discontinue the development of ACP-044 in acute and chronic pain based on an evaluation of the final dataset from the bunionectomy surgery Phase II trial. ACP-319, an M1 PAM modulator, based on a profile that does not support advancement to Phase II has also been dropped. With the ADP uncertainty removed and the program re-prioritization (dropped ACP-044 & ACP-319), we belief investors will appreciate the renewed focus. In our view, the prioritization is a good business decision and should both help the share price and attract potential buyers to ACAD as the Nuplazid revenue stream and focused pipeline would be a nice fit with many Pharma/Big Bios out shopping for bolt-ons. Nuplazid is annualizing over $500 million a year in sales and the Baker Brothers still hold about 25% of the stock.
BCYC — Provides Q2 Update, Data for BT8009 & BT5528 in 2022
BCYC continues to make clinical progress with both BT8009 and BT5528. Both of the lead drug development candidates will have data readouts later this year. The BT8009 data (in H2:22) will inform on the new dosing cohorts which should improve upon the data reported earlier this year at AACR. In Q3, the company will have Phase I BT5528 data and positive results would allow for development in ovarian cancer and potentially other oncology indications.
BT8009: The next data update for BT8009 is anticipated in H2:22. Bicycle will present results from the new cohorts (10 mg/m2 Q2W and 7.5 mg/m2 (2 on, 1 off) dosing frequencies) in addition to more mature data from prior cohorts (5 mg/m2 QW, 2.5 mg/m2 QW, and 7.5 mg/m2 QW).
BT5528: Additional data readout from the ongoing Phase I trial is expected in Q3:22. The trial enrolled 24 patients and data from 14 patients have been presented thus far. In addition to new data, we expect more mature data from early enrollments. The company also announced the initiation of Phase II expansion cohort with 56 patients in June. BCYC expects to announce topline data followed by more detailed presentation at a medical conference.
BCYC has significant cash resources and ended Q2 with $3723 million in cash and expects this to fund operations through 2025. In our view, the selloff at ASCO was way over done and the stock has bounced nicely after the bear raid. The company will deliver data for both BT8009 and BT5528 later this year which could serve as nice catalysts for the stock.
BMRN —BMRN Reports Strong Voxzogo Launch Revenue
BMRN recently reported financials with 2Q total revenue of $533.8 million (+3% QoQ; +6% YoY) which was slightly below the consensus estimate of $52 million. Revenue was negatively impacted by COVID and the timing of ex-US orders. The Voxzogo launch continues to be strong with 2Q revenue of $34.4mn (+75% QoQ) which easily beat the consensus estimate of $27 million. The strength has caused BMRN to raise their FY22 guidance for Voxzogo to $130-$160 million (vs. $100-$125million prior) on favorable demand trends and geographic expansion. Management also discussed potential avenues for label expansion and have had ongoing FDA discussions regarding Phase II data in younger patients which are expected in 2H.
Given the strong Voxzogo launch, our confidence is growing in the potential for Roctavian’s outlook in Europe after a positive regulatory opinion from the EMA. An EU approval expected in late-August and given the clinical profile we are cautiously optimistic on the path forward in the US, where the BLA resubmission is on track for September. Management highlighted the recent approval without a lower age limit in Japan, which BMRN sees as a key market given the large population (~2/3 of the estimated 1.5K patient opportunity in the APAC region).
Medicate Impact
BMRN expects minimal impact from Medicare for the following reasons:
- 60%+ of revenue is ex-US, and 76% of the remaining ~40% of US-based revenue is from commercial payors;
- BMRN’s products and late-stage pipeline drugs fall under the provisions’ exclusions of single-indication orphan drugs (<$200mn in Medicare spending) and drugs with generic or biosimilar competition;
- Inflationary rebates on Medicare Part B and D should not be triggered if BMRN remains consistent with historical pricing trends.
CLDX — Q2 Call All CDX0159 Trials Intact And On Time – Two New Papers Highlight Breadth of MAST CELL & Bi-Specific Ab Opportunity BUY
CLDX’s quarterly release highlighted its lead mast cell antibody CDX-0159 and the broad clinical program underway. We also just came across two recently published peer reviewed articles – one on mast cells that implies very broad potential for ‘0159 in bone diseases like osteoporosis; the other is on CD27 inhibition that has positive implications for Celldex’s novel bi-specific antibody, CDX-527. REITERATE BUY
Barzolvolimab (CDX-0159) Four Studies So Far – Two urticaria trials utilizing the subcutaneous formulation are underway – CSU and CindU.
- A Study of CDX-0159 in Patients With Chronic Inducible Urticaria – patients recently began enrollment –
https://clinicaltrials.gov/ct2/show/NCT05405660?term=Barzolvolimab&draw=2&rank=2
- A Phase 2 Study of CDX-0159 in Patients With Chronic Spontaneous Urticaria – this study also recently began enrollment
https://clinicaltrials.gov/ct2/show/NCT05368285
- A Study of CDX-0159 in Patients With Prurigo Nodularis – enrollment is underway since late-2021
https://clinicaltrials.gov/ct2/show/NCT04944862?term=Barzolvolimab&draw=2&rank=3
- A Study of CDX-0159 in Patients With Eosinophilic Esophagitis (EoE) – The Phase II trial in eosinophilic esophagitis (EoE), the most common type of eosinophilic gastrointestinal disease, is due to start enrollment in Q4:22.
We know one thing is certain – CDX-0159 use to treat both CindU and CSU retests in rapid, profound and durable responses across multiple dosing groups with favorable safety profile. The SubQ version has identical parameters but may have the following advantages over the IV version – a) potential for at home/sefl administration use or use in a doctor’s office (versus an infusion center); b) possibly an improved safety profile .
Mast Cells Impact On Osteoblasts
A study was just published (July 28) in the peer-reviewed Matrix Biology entitled, “Mast Cell Chymase Has a Negative Impact on Human Osteoblasts” (Matrix Biol https://pubmed.ncbi.nlm.nih.gov/35908613/). The article details how “mast cells have been linked to osteoporosis and bone fractures, and in a previous study….found that mice lacking a major mast cell protease, chymase, develop increased diaphyseal bone mass.” In the study, the authors conclude that the present findings provide a functional link between mast cell chymase and osteoblast function, and can form the basis for a further evaluation of chymase as a potential target for intervention in metabolic bone diseases. In our view, CDX-0159 broad and unequaled mast cell inhibition might lead to yet another blockbuster indication down the road.
Drugs In The Reconciliation Transformation: Inflation Reduction Act of 2022 – Why It Makes CDX-0159 More Valuable
The largest drug company argument against the new drug pricing law is that drug companies spend billions of dollars and a decade getting a new drug approved and barely recoup the investment, at least until the 9 year negotiation period expires which the new Medicare deal lays out.
The ability to add on additional indications, pipeline in a pill, leads to the largest returns AFTER the new indications are funded and then approved as each new indication is price protected for 9 years. Many new blockbuster drugs (MRK’s Keytruda, ABBV’s Humira etc….) are approved for 20-30 different indications. Hence, we believe that an effective and safe mast cell inbibitor like CDX-0159 is an ideal candidate for the broad clinical development plan that is already underway. With the studies above plus the new papers that keep getting published, we think the compound (and hence Company) is more valuable than ever. One day we are confident that Celldex will be taken out at a large premium.
Another Article Is Positive For CDX-527
A intriguing new study was just published in the July 20 edition of the European Journal of Immunology titled, “Agonistic anti-CD27 antibody ameliorates EAE by suppressing IL-17 production” (https://pubmed.ncbi.nlm.nih.gov/35856659/). The abstract explains that CD27/CD70 costimulation enhances T-cell survival, memory formation and Th1-cell differentiation and effector function. In addition to promoting Th1 responses, CD27 signaling has been shown to exert a negative regulatory role on IL-17 production, resulting in increased sensitivity of CD27 KO mice to experimental autoimmune encephalomyelitis (EAE). As a bispecific antibody, CDX-527 is a combination both CD27 and PD-1 inhibitor. CDX-527 is the first candidate developed by Celldex from its bispecific platform which utilizes the Company’s proprietary highly active anti-PD-L1 and CD27 human antibodies to couple CD27 co-stimulation with blockade of the PD-L1/PD-1 pathway. In the Phase 1 dose-escalation study of CDX-527 in patients with advanced or metastatic solid tumors that have progressed during or after standard of care therapy, enrollment to the dose escalation portion of the study has been completed and an expansion cohort in ovarian cancer is currently enrolling patients. Hence, while the initial trials are in cancer, CD27 is sound to its natural ligand CD70, and the potential in autoimmune is also there. This is one is way off the radar screen for CLDX investors, but does show that Celldex has some exciting new compounds behind ‘0159, too.
Well Financed To The End of 2025
CLDX ended Q2 with $356 million in cash and no debt, good until the end of 2025. Hence, no need for additional dilutive financing. They are in sound financial shape.
Still one of our very favorites at current levels, CLDX is a BUY under 55 with a TARGET PRICE of 85.
ESPR — All CLEAR As Important Outcomes Trial Reaches 100% Events, Data Remains on Track for Q1:23
ESPR recently reported a slight Q2:22 miss that included US Nexletol/Nexlizet revenue of $13.6 million, the +1.7% QoQ growth and a little below the consensus estimate of $14.7 million. Sales came in marginally lower in part due to a Medicare coverage gap that the company paid during the quarter and the initiation of a Medicare coverage contract with Cigna. Sales continue at a steady growth rate but investors are CLEARly focusing on the upcoming Outcomes trial; CLEAR (https://clinicaltrials.gov/ct2/show/NCT02993406?term=Esperion&draw=2&rank=8).
Importantly, ESPR announced that the CLEAR Outcomes trial has reached 100% of accumulated events, meaning that the targeted 1,620 primary major adverse cardiovascular events (MACE-4) have been reached. Additionally, as previously reported, the study has also reached >100% of the targeted 810 MACE-3 events (CV death, non-fatal MI, non-fatal stroke). In our view, CLEAR will read out positive due to the drug’s ability to significantly reduce LDL and will grow Nexletol/Nexlizet sales substantially.
Our confidence is based on a combination of previously positive de-risking clinical data and the Company’s experience in cardiovascular drug development, specifically large trials like CLEAR. ESPR also retains U.S. rights making it more valuable in an acquisition which could come even before CLEAR, even though most potential suitors will want to wait for CLEAR.
As a once-daily alternative mode of action to other cholesterol lowering drugs like statins, we believe ESPR’s drug fills and important void (statin intolerant patients) that others players in the CV space will desire once the data is released. We are getting much closer to that event and the Company is laser focused and as the call above says, is right on time. BUY
INCY — Reports Solid Jakafi Revenue, Opzelura Comes in Light Due to Initial Sampling
INCY provided 2Q results with solid Jakafi sales. 2Q total product and royalty revenue of $781 million was slightly below the consensus estimate of $807 million. The miss was primary caused by the unfavorable impact of foreign exchange rates on royalty revenues. Jakafi 2Q net sales of $598 million were above the $591 million consensus estimate. INCY also further tightened Jakafi FY guidance to $2.36-2.40 billion (vs. $2.33-2.40 billion updated in 1Q). The company continues to manage the Jakafi patent cliff with growth driven by demand across MF, PV and GVHD with new patient starts remaining above pre-pandemic levels. The GVHD patient market is important and going well at 18% y/y with a strong launch and now represents 15% of Jakafi net sales.
The Opzelura launch remains our primary focus with the recent approval in vitiligo representing a large and previously unmet need. The Q2 sales of $16.6 million were below the consensus estimate of $20 million with script data showing the monthly script trajectory decelerating. On the conference call management noted the deceleration was due to the shift from free drug to formulary access, representing a “transitional quarter.”
The shift also created an administrative burden to pharmacies, causing temporarily delayed filling of prescriptions. INCY is working closely with pharmacies to address the evolving fulfillment process. The company noted that Opzelura demand was strong in late July, alleviating concerns of the quarterly miss. In our view, it is early days for the Opzelura launch and the drug is poised to become a blockbuster topical/derm drug. We suggest subscribers take advantage of the stock sell off due to temporary and likely already corrected initial ordering/prescriptions of Opzelura to due initial free samples.
IONS — 2Q Financials, Eplontersen Data at ISA Sept. 4-8
IONS reported Q2 financials and Spinraza royalties came in a bit light at $60 million vs. the consensus estimate of $63 million. IONS also reiterated FY22 revenue guidance of $575 million vs. the consensus $586 million. While Spinraza continues to face competitive pressure from both (NVS’ Zolgensma and ROG/PTC’s Evrysdi) – not new news. In our view, BIIB/IONS are managing the situation well and continue to deliver solid revenue growth while also addressing the competitive situation. BIIB recently reported positive data from the Phase IV trial RESPOND which is testing Spinraza in SMA patients who have has a suboptimal response to gene therapy (and come back to Spinraza).
Moreover, the focus of IONS shares has shifted to the late-stage pipeline. After the recent positive data from ALNY’s Onpattro – which also boosted IONS in sympathy – we are looking forward to IONS’ detailed Phase III eplontersen data in hATTR-PN at the upcoming International Symposium on Amyloidosis (ISA) meeting (Sept.4-8). This compound is being developed by IONS and partner AstraZeneca.
Investors will be looking at the study’s co-primary endpoints of neurological impairment and TTR reduction that were both met in June of this year. An NDA is expected in H2, to be followed by filings in the EU. In our view, the drug candidate could compare favorably to ALNY’s Onpattro by demostrating both TTR reduction and mNIS+7 improvements which indicates a reversal of neurological impairment symptoms. The eplontersen data has the potential to move the needle for IONS at ISA Sept. 4-8. As a reminder, ALNY market cap has risen over $10 billion since it’s positive data was announced (+65%). IONS’s has risen just 24% or about $1 billion. We know eplontersen worked in Phase III – when the market sees the actual positive data and can compare it with Onpattro, IONS shares could rally much further.
MDGL — MAESTRO-NASH Remains on Track for Q4
In the Q2 quarterly release, MDGL announced that resmetirom remains on track to deliver topline data from the pivotal MAESTRO-NASH (n=2,000) biopsy study (n=2000) in the fourth quarter. These studies are intended to serve as the foundation for a subpart H NDA submission for resmetirom in the non-cirrhotic NASH population in the first half of next year. In our view, the positive Phase III data from MAESTRO-NAFLD (n=972) significantly de-risks the upcoming Phase III data read out in NASH. The NAFLD data demonstrated that resmetirom is safe and well-tolerated at 80mg and 100mg QD dosing in patients with NAFLD treated for 52-weeks. Resmetirom has shown the ability to significantly reduce both liver fat and atherogenic lipids. In our view, reductions in key CV-risk measures including LDL-C, ApoB, and triglycerides make this a best in class drug in NASH.
In June MDGL launched the ‘NASH Explored’ disease education campaign for healthcare providers and announced expanded partnerships with key patient advocacy groups. Additionally, the Market Access and Medical Affairs teams have conducted NASH disease education sessions with payers that together account for more than 80% of all branded prescriptions in the U.S.
The company is clearly confident that MAESTRO-NASH will read out positive given the positive Phase III NAFLD data. We also believe, based upon the totality of the clinical data to date, that the trial will be successful and that resmetiron will be the very first NASH drug approved and set the standard for both clinical, therapeutic and commercial development in the nascent yet potentially blockbuster field.
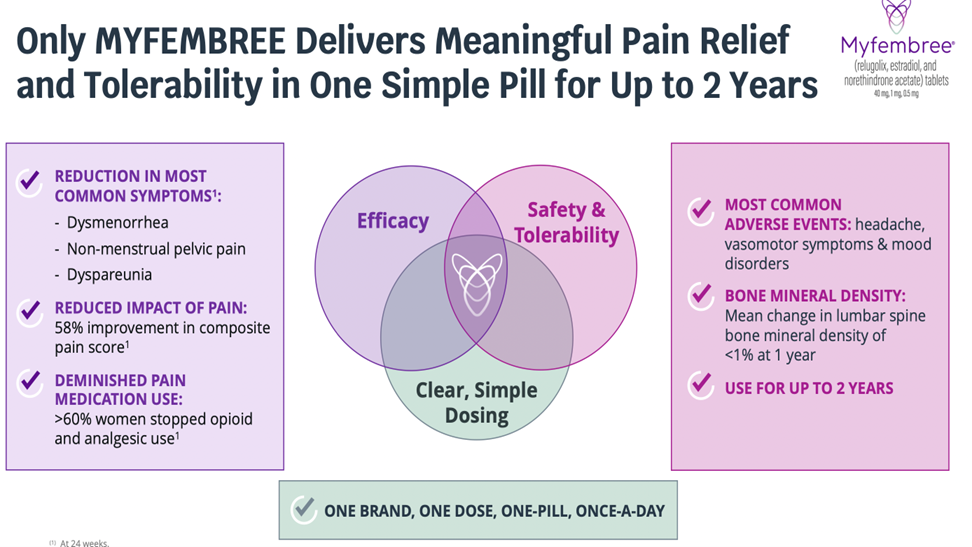
MYOV — FDA Approves Myfembree For Endometriosis Pain; Significant Growth Ahead Plus Triggers $100 Million Payment From Pfizer
Right at the PFUFA date FDA approved the sNDA for Myfembree (relugolix) in moderate-to-severe pain associated with endometriosis (EM). With the third indication for relugolix (prostate cancer, uterine fibroid (UF) and now endometriosis), Myovant is delivering what we believe is a class leading GNRH antagonist for male (branded as Orgovyx and female (branded as Myfembree) reproductive health. The Orgoxyx launch has been very successful to date, with the Myfembree launch less so although it is still the leader in the new patients adds in UF. With the EM indication we expect Myfembree sales to accelerate almost immediately, as there is almost complete overlap of the targeted physician group for EM as it has for UF. BUY
All Clear In EM; $100 Million From Pfizer
There were concerns back in April when the FDA had additional questions for the EM indication – leading some to doubt its potential approval (as many late-stage compounds in biotech had received CRLs in the past year). However, the label is as clean and as broad as expected. We believe that growth will follow based on the drug’s once-daily (vs. 2x/day for the competition) and its major advantage of less bone marrow loss. The product is available immediately – with an official launch due in late-August. Also, not insignificantly the approval triggers a whopping $100 million milestone payment from Pfizer.
Myovant management sees many synergies with the ongoing Myfembree uterine fibroids launch that should help them immediately access the expanded market opportunity with efficiency. In terms of absolute size of the markets, populations of 5M/6M patients with UF/EM, respectively, 3M and 1M of whom are failed by initial treatments.
First quarter Myfembree sales were only $4 million in the fourth full quarter of launch, however, for several reasons we expect the women’s health revenues are about to take off.
- First in June 2022, the FDA accepted for review an sNDA that proposes updates to MYFEMBREE’s U.S. Prescribing Information (USPI) based on 2-year safety and efficacy data from the Phase 3 LIBERTY randomized withdrawal study (RWS) of MYFEMBREE in premenopausal women with heavy menstrual bleeding associated with uterine fibroids. The FDA set a target action date of January 29, 2023 for this sNDA. That will make the UF label more competitive.
- In June 2022, Myovant and Pfizer announced that the results of the Phase 3 SPIRIT 1 and SPIRIT 2 studies of once-daily relugolix combination therapy (relugolix 40 mg, estradiol 1.0 mg, and norethindrone acetate 0.5 mg) in women with endometriosis-associated pain were published in The Lancet.
- Additional data from the SPIRIT 2-year extension study in women with endometriosis were presented at the European Society of Human Reproduction and Embryology (ESHRE) 2022 Annual Meeting in July 2022. The Society recognized the presentation as the best oral presentation of a clinical topic at the ESHRE 2022 Annual Meeting.
- The now-expanded label in endometriosis pain will give the sales force plenty of ammo to emphasize the convenience of the one-pill, once-daily regimen to physicians.
- Management also noted that payer discussions are ongoing, and there is just currently 18% coverage.
Hence, we believe that Myfembree has huge growth potential. With PFE using some of its COVID cash to buy bolt on companies of late (e.g., GBT for $5 billion this week), now that the EM approval is official we would not be surprised if they take out MYOV, too.
PGEN — Q2 Update: Executing At A High Level, Enrollment Complete for PRGN-3006 (AML), PRGN-3005 (ovarian cancer), PRGN-2012 (RRP) & PRGN-2009 (HPV cancers)
PGEN’s stock has been quite strong as the combination of a strong clinical update with four trials completing enrollment and also a positive read through from the recent Roche/Poseida deal. PGEN is particularly excited for the Phase I data presentation for the PRGN-2012 AdenoVerse study in Q4:22. BUY
PRGN-3006 – Enrollment is complete in Phase 1 study of PRGN-3006 UltraCAR-T® in acute myeloid leukemia (AML); enrollment ongoing in Phase 1b dose expansion study; Mayo Clinic in Rochester, Minnesota activated as first expansion site of Phase 1b multicenter expansion; technology transfer and site activation activities underway at multiple new sites.
PRGN-3005 – Enrollment complete in Phase 1 study of PRGN-3005 UltraCAR-T in advanced ovarian cancer; enrollment complete at Dose Level 3 with lymphodepletion in the IV arm; Phase 1b expansion study initiated at Dose Level 3 with lymphodepletion prior to IV infusion; technology transfer and site activation underway for Phase 1b multicenter expansion.
PRGN-2012 – Enrollment complete in Phase 1 study of PRGN-2012 AdenoVerse™ Immunotherapy in recurrent respiratory papillomatosis (RRP); Phase 2 study initiated and rapidly progressing.
PRGN-2009 – Enrollment complete in combination arm of Phase 1 study of PRGN-2009 AdenoVerse Immunotherapy in human papillomavirus (HPV)-associated cancers.
Balance Sheet Addressed – More To Come
PGEN ended the quarter with $130 million in cash. The Company just raised an additional $170 million in non-dilutve cash from a sale of a non-core asset, alleviating any concerns of the mid-2023 upcoming convertible debt due date. We expect more to come plus at least one large corporate partnership with one of the four compound above.
Since the last Issue, a large cancer deal between Roche and Poseida (up over 70%) for CAR-T therapeutics was formed – and has boosted both PGEN and TCRT as investors realized that both companies were significantly undervalued relative to Poseida. Roche gave PSTX $110 million in cash upfront and they also raised another $70 million in a public offering. We expect PGEN will form at least one deal like this in the near-term. Still one of our very favorite small cap names and they are executing at a very high level.
SGMO — Remains on Track, Fabry Data at SSIEM in September
Sangamo continues to make progress across four major clinical programs, plus there is a large number of preclinical programs and top tier partnerships (PFE, Roche, AZN, BIIB, etc) that in this environment could lead to a buyout at any time. While many are in competitive therapeutic/technology programs, three of the four are still wholly-owned programs. Still way undervalued in our view, SGMO is a BUY.
Fabry disease – Received endorsement to progress into the Phase I/II trial expansion; continued to recruit patients and activate sites; Phase III planning progresses.
- A total of 16 study sites are now open and recruiting, including the first sites in Canada,Italy and Australia. Expect to dose two additional patients imminently, and have multiple patients in screening, including both male and female candidates. Continue to actively prepare for a potential pivotal Phase III trial.
Sickle cell disease – Completed transition of program back to Sangamo; advanced manufacturing activities in anticipation of dosing in Q3; Phase III planning progresses.
- Dosing of the next patient is anticipated in the third quarter of 2022. Phase III enabling activities and manufacturing readiness are in progress.
Hemophilia A – Pfizer expects resumption of dosing in Q3 2022; pivotal data read-out expected in late 2023 or early 2024.
- A pivotal data readout is estimated in late 2023 or early 2024 with over 50% of the patients have been enrolled in the Phase III AFFINE trial.
Renal Transplant Rejection – Received Orphan Medicinal Product Designation from the European Commission; progressed manufacturing and clinical activities ahead of anticipated Q3 dosing.
- Completed manufacturing of the dose for the second patient, who recently received a kidney transplant. Dosing of this second patient is expected later in the third quarter of 2022. Plan to complete dosing of the first cohort, comprised of three patients, by the end of 2022.
Prion 2022, September 13-16, 2022, Gottingen, Germany
Upcoming Medical Conferences
- Society for the Study of Inborn Errors of Metabolism (SSIEM), August 30-September 2, 2022, Freiburg, Germany Updated Fabry results from the STAAR study
SGMO continues to advance their pipeline with Fabry data at SSIEM an upcoming potential catalyst. The recent side effect issues with elevated liver enzymes at Intellia (NTLA) remind us about the potential problems with CRISP technology. While the elevated liver enzymes are probably not a serious issue as they occur in many drug development programs, the stock sold off substantially as investors remain wary of side effects in CRSPR technology stocks. In our view, SGMO remains undervalued with a proprietary gene and cell therapy editing platform (zinc fingers) that is both safe and patent protected.
VXRT — Q2 Update COVID and Norovirus Oral Vaccines
Vaxart has begun working on vaccine candidate constructs that directly target new omicron variants of concern and selection of a specific vaccine construct will be chosen in Q4 with clinical trials to start in 1H 2023. Recent FDA guidance for new COVID-19 vaccine boosters is that they should target omicron BA.4/5. However, the vaccine leaders will have booster data this quarter and VXRT will not have booster data until next year as the company never seems quite able to catch up. The Company also is preparing to release top-line data in the third quarter from its Phase II COVID-19 testing of a Wuhan strain vaccine construct. The value of Wuhan strain data is hard to factor when the FDA is currently focusing on new booster data that targets omicron BA.4/5. But investors need to see that Vaxart has positive human COVID oral vaccine data – regardless of the strain for now. A positive human trial will bode quite well for future constructs, because we have only seen published preclinical results.
VXRT plans to start a Phase II trial of their bivalent norovirus vaccine candidate in the fourth quarter. The Company will follow up with top-line data from their ongoing Phase II norovirus challenge study at the end of Q1/23/early Q2/23 which could serve as a potential catalyst. Data from their noravirus trial in elderly adults has been encouraging, as it suggests similar activity to that in younger adults, which is often not the case with injectable vaccines. The Company ended the quarter with over $130 million in cash.
ZYNE — ZYNE Reports Q2 Financials
ZYNE recently announced financial results for the second quarter indicating a $9.9 million quarterly cash burn rate and $62.5 million in cash and equivalents. On July 21 the company announced a share purchase agreement with Lincoln Park Capital for up to $20 million which provides a cash runway into 2024, past the anticipated release of the Phase 3 RECONNECT trial data for Zygel in Fragile X Syndrome (FXS).
That data is expected in the second half of 2023. In the quarterly release ZYNE emphasized their focus on completing RECONNECT, which is the second Phase 3 trial for FXS. The company is currently recruiting patients with a methylated FMR1 gene, the patient subgroup which experienced the best results in the previous CONNECT-FX Phase 3 trial. Following promising Phase 2 trial released in June, ZYNE will also continue development of Zygel in 22q11.2 Deletion Syndrome (22q).
The next milestone for that program will be a meeting with the FDA, which has not yet been scheduled, to discuss next steps. Considering currently limited cash resources and the promising data in FXS and 22q, ZYNE reiterated plans to put the next development steps for Zygel in Autism Spectrum Disorder (ASD) on the back burner.
The Back Page
| Symbol | Company | Orig.Rec. | Current | Target | Recommendation |
|---|---|---|---|---|---|
| ACAD | Acadia | 33.79 | 15.74 | 45 | BUY under $28 |
| ALKS | Alkermes | 10.13 | 25.47 | 55 | BUY under $35 |
| BCYC | Bicycle | 43.92 | 25.91 | 75 | BUY under $50 |
| BMRN | BioMarin | 12.68 | 95.07 | 150 | BUY under $100 |
| CLDX | Celldex | 10.50 | 34.53 | 85 | BUY under $55 |
| ESPR | Esperion | 24.42 | 6.64 | 25 | BUY under $10 |
| INCY | Incyte | 5.88 | 73.76 | 100 | BUY under $80 |
| IONS | Ionis | 7.63 | 43.44 | 65 | BUY under $50 |
| MDGL | Madrigal | 17.00 | 70.82 | 275 | BUY under $200 |
| MYOV | Myovant | 13.74 | 17.70 | 45 | BUY under $30 |
| PCRX | Pacira | 15.78 | 56.25 | 100 | BUY under $80 |
| PLXP | PLx Pharma | 16.70 | 2.59 | 12 | BUY under $6 |
| PGEN | Precigen | 34.42 | 2.32 | 24 | BUY under $12 |
| SGMO | Sangamo | 4.77 | 5.82 | 30 | BUY under $20 |
| TCRT | Alaunos | 8.00 | 1.78 | 12 | BUY under $5 |
| VXRT | Vaxart | 8.00 | 4.07 | 30 | BUY under $15 |
| ZYNE | Zynerba | 8.00 | 1.30 | 16 | BUY under $8 |
*New recommendation.
THE MODEL PORTFOLIO*
| COMPANY | SHARES OWNED | TOTAL COST | TODAY’S VALUE |
|---|---|---|---|
| Long Positions | |||
| Acadia | 4,750 | 156,557 | 74,765 |
| Alaunos | 26,125 | 166,100 | 46,502 |
| Alkermes | 3,800 | 88,690 | 96,786 |
| Bicycle | 2,400 | 105,408 | 62.184 |
| Celldex | 15,832 | 174,993 | 546,678 |
| Esperion | 3,316 | 105,316 | 22,018 |
| Incyte | 1,229 | 34,817 | 90,651 |
| Ionis | 3,087 | 49,123 | 134,099 |
| Madrigal | 3,127 | 69,980 | 221,454 |
| Myovant | 7,125 | 103,853 | 126,112 |
| Pacira | 2,375 | 63,887 | 133,617 |
| PLx Pharma | 15,359 | 269,988 | 39,779 |
| Precigen | 9,690 | 76,510 | 22,480 |
| Sangamo | 19,456 | 253,596 | 113,233 |
| Vaxart | 29,687 | 250,000 | 120,826 |
| Zynerba | 10,192 | 150,003 | 13,250 |
| (08/11/22) | Equities: | $1,864,236 | |
| Cash: | $27,796 | ||
| PORTFOLIO VALUE: | $1,892,236 |
*The Model Portfolio is designed to reflect specific recommendations. We began the Model Portfolio on 12/23/83 with $100,000. On 4/13/84, we became fully invested. All profits are reinvested. Stocks recommended since then may be equally attractive, but may not be in the Model Portfolio. Transactions and positions are valued at closing prices. No dividends are created, and we don’t use margin. Interest income is credited only on large cash balances.
The Model Portfolio
BENCHMARKS
| NASDAQ | S&P 500 | MODEL | |
|---|---|---|---|
| Last 2 Weeks | 3.04% | 1.8% | 11.4% |
| 2022 YTD | -18.30% | -11.7% | -16.9% |
| Calendar Year 2021 | 21.3% | 26.9% | -15.2% |
| Calendar Year 2020 | 43.6% | 16.3% | 13.8% |
| Calendar Year 2019 | 35.2% | 28.8% | 10.7% |
| Calendar Year 2018 | 5.7% | 6.6% | 4.5% |
| Calendar Year 2017 | 29.3% | 19.9% | 65.6% |
| Calendar Year 2016 | 7.5% | 9.5% | -29.6% |
| Calendar Year 2015 | -0.1% | -0.1% | 25.1% |
| Calendar Year 2014 | 13.4% | 11.4% | 29.2% |
| Calendar Year 2013 | 38.3% | 29.6% | 103.4% |
BENCHMARKS
New Money Buys
NEW MONEY BUYS
Contact Info
Medical Technology Stock Letter
John McCamant, Editor
Jay Silverman, Editor
Jim McCamant, Editor-at-Large
BioInvest.com
579 Mangels Ave.
San Francisco, CA 94127
510-843-1857
Send us an email
Download a PDF of MTSL Issue #982
©Piedmont Venture Group (2022). Address: 579 Mangels Ave., San Francisco, CA 94127. Telephone: (510) 843-1857. Fax: (510) 843-0901. BioInvest.com. Email: admin@bioinvest.com. Published 24 times a year. Email subscription rates: 1 year – $399; 2 years – $678; 3 years – $898. You may cancel at any time for a prorated refund. The information and opinions contained herein have been compiled or arrived at from sources believed to be reliable but no representations or warranty, express or implied, is made as to the accuracy or completeness. In no way shall this newsletter be construed as an offer to sell or solicitation of an offer to buy any securities. The publisher and its associates, directors or employees may have positions in, and may from time to time make purchases or sales of, securities mentioned herein. We cannot guarantee and you should not assume that future recommendations will equal the performance of past recommendations or be profitable.