MTSL Issue 975
IN THIS ISSUE: Surviving A Biotech Bear Market
Since Last Issue: BTK: -9.2%; NBI: -8.9%; XBI: -13.2%; Model Portfolio: -10.4%; Trader’s Portfolio: -30.4%
SENTIMENT — Economics 101: Rates Way Up, Biotech Stocks Way Down
COVID News: Spring Spike As Outdoor Life Resumes, Europe/China Not Making It Any Easier; Boosters/Antivirals Still Avoid Worst Cases; Where Is NVAX? JNJ, GSK Show Solid COVID Sales
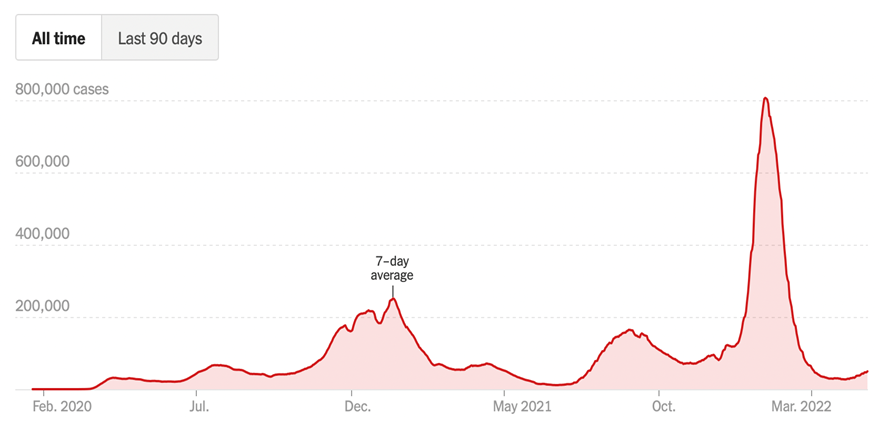
Overall The U.S. Doesn’t Look Bad But…..
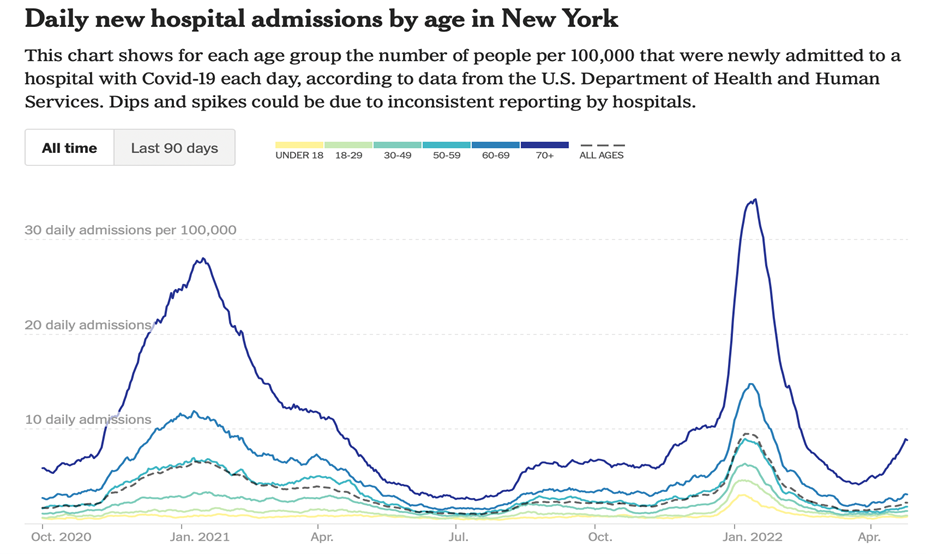
Positive cases are up 53% over the past two weeks, led by the Northeast. Deaths (-32%) and hosptializations (+4%) have declined overall but there is a lag between the positive infections and the worst outcomes (hosp/death) so one can expect to see the very bad new cases rise again, at least a little. Testing is up 8% but with the spike in new cases, look for more testing to be needed, too. The second chart above in NY shows the larger rise in daily hospital admissions due to COVID, and the CDC is recommending mask mandates in many counties. The warmer weather is leading to a resumption of large gatherings again (e.g., concerts, religious, etc.) and that is often led by un-boosted or not vaccinated people. So we have ongoing COVID concerns, not to mention the increasing long COVID sufferers.
China Spiking & So Is Europe
Turning to the ROW, it really is not a pretty picture. The lockdown of Shanghai is only adding to the slowdown in global trade and hence, inflationary implications. The U.S. spike looks relatively light compared with Germany, France, Italy and South Korea. So COVID – often forgotten with the Ukraine crisis – is a major reason that the U.S. is raising interest rates to slow down out of control prices.
Second Booster Recommendations Widening
An FDA advisory panel last week supported extending boosters. Even though the ba.2 variant is overall a milder version, we are still learning a lot about its virulence. Obvious symptoms – cough, fever, aches/pains – are being dismissed as minor but the inflammatory and autoimmune components are starting to emerge for longer term disability. While the vaccines haven’t stopped the virus dead in its tracks like we all had hoped they might, vaccines prevent the worst outcomes of hospitalization and death. So getting a second booster after 4-5 months is a smart move. Vaccine stocks are hanging in there for now (except NVAX which is still not FDA approved).
NVAX EUA Still Elusive
Despite positive clinical data and approval in some overseas countries, the long underperformance of Novavax continues with a COVID vaccine that is nowhere in sight in the U.S. Not that we need another vaccine at this point, but NVAX was one of the early frontrunners in the race and a recent FDA AdCom pointed out that it is still not entirely sure when any EUA will come.
Both JNJ, GSK Deliver Solid COVID Performances
With both companies somewhat under the radar (vs. PFE and MRNA), it shows that the use of COVID vaccines and antibodies is not just limited to the first two vaccines. JNJ’s Janssen vaccine and GSK’s antibody (Xevudy) released positive, larger than expected revenues in their latest quarterly reports.
SENTIMENT – Rates UP, Stocks DOWN
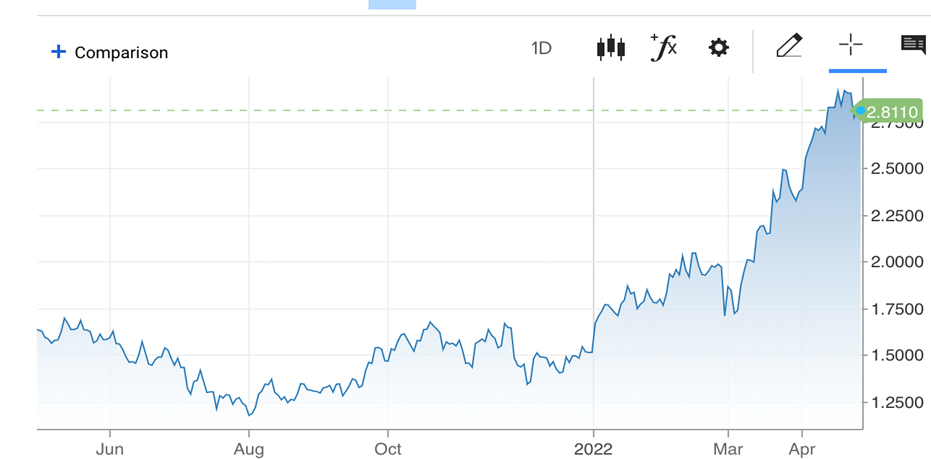
The basics are very simple. When rates go up, stocks go down. More specific to biotech, when rates go way up, risky stocks go way down. We have put long bond yields in the MTSL this year to show this effect. One cannot fight the Fed and this type of very aggressive Fed in particular is faced with the consequences of long overdue free money stoking inflation that has run wild. As they pull that extended safe rug out from under investors, we really have seen that there is no place to hide. The wealth disparity has widened to unreasonable levels by any measurement, with the implications of the Elon Musk takeover of Twitter for $44 billion alone shows how extreme things have become. On top of that, the Fed is in a bind with too many variables that are out of its control (e.g., Russia, supply chain and ongoing COVID spikes to name a few) and has to resort to major interest rate hikes to stop runway prices. That just hurts risk assets regardless, and really hurts unprofitable, risky stocks like the bios.
As long term biotech analysts, one has to be an optimist on at least a handful of companies with encouraging compounds and improving fundamentals. This week’s great example on the plus side is Alkermes (ALKS) and BioMarin (BMRN), two MTSL Recommendations that delivered the goods in broad overall quarterly beats that even the shorts can’t argue with. Frankly speaking, the majority of biotech companies are coming up short lately – especially at the FDA, the industry’s most crucial gatekeeper. Earlier this year we wrote of increasing “green shoots” with positive data updates and even an FDA approval or two. There was select good news for BTAI but one of the sector leaders, ALNY, received a 3-month delay for vutrisiran. Other recent FDA pushbacks from AXSM and MYOV are just a sample of the longer list that has many thinking the FDA is becoming a lot tougher than it has been in a while.
Surviving The Biotech Bear – Something’s Gotta Give
When there are good company catalysts (e.g., data, FDA approvals, collaborations, etc.), they are only met with “sell on the news” action and/or immediate dilutive financings in almost every instance. Since the last Issue, after the two recent takeovers (SRRA/GSK, HALO/ATRS) there was another nice small deal ($225 million) of a checkpoint oncology company, when CMPI was bought by Regeneron for almost 4x the previous days close. But even that impressive premium has not been enough to stop the massive exodus of risk in portfolios. Also this past week, shares of NKarta (NKTX) more than doubled and rose even further after rising $180 million in a follow on after releasing the Company’s off-the-shelf treatments, both made from engineered natural killer cells, induced complete responses (CRs) in patients with advanced forms of blood cancer. Both the CMPI takeover and the NKTX data (and the BGNE/NVS data mentioned below) are very positive signs for biotech but not enough to change the sector’s (and market’s) negative momentum.
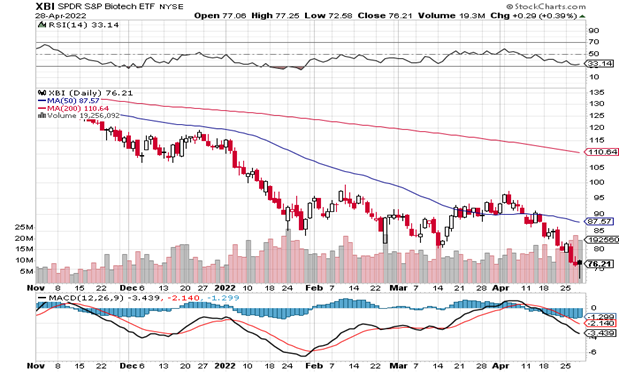
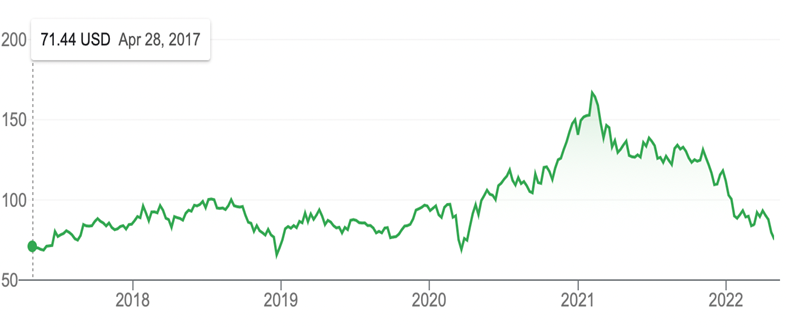
More than offsetting the good ALKS and BMRN news, sector leaders AMGN and ALNY released overall quarterly disappointments – the former due to a widening long-term tax fight with the IRS. The real sad part, however, is that we are still not yet at oversold conditions based on the RSI (33). It just feels like we’ve been oversold for over a year now. The chart below is one of the more depressing ones we have encountered – the XBI (77) is only slightly higher than it was 5 years ago. Every up morning is greeted with a down end of the day – the worst technical action possible. The only way to look at is that it’s going to take time and when even the mighty Powell cannot tame the inflation beast, little biotechs are just rounding errors until an easing monetary policy change begins.
Where Are The Blockbusters?
In our decades analyzing biotech companies the industry experienced many periods of fruitful successes in the form of not just FDA approvals but drugs that subsequently turned into blockbuster drugs. The COVID vaccines from MRNA and BNTX/PFE are bonafide money makers but looking around at the landscape of late such wins are few and far between. While it doesn’t seem that way at all based on the stock market, there are some fundamental winners out there. Just this this, oncology based BeiGene (BGNE) and partner Novartis (NVS) announced that their investigational cancer drug tislelizumab succeeded in meeting the primary endpoint of overall survival in a global Phase III trial. Tislelizumab is a humanized IgG4 anti-PD-1 monoclonal antibody that helps to minimize binding to Fc-gamma receptors on macrophages to support the immune cells detect and fight tumors. Data from the RATIONALE 306 study demonstrated tislelizumab’s ability to improve survival outcomes for patients with previously untreated advanced or metastatic esophageal squamous cell carcinoma when combined with chemotherapy. No one is paying for any future successes right now and it is our job at MTSL to find these harder to find needles in a much bigger haystack. We think we have two more potential blockbusters in MDGL’s resmetirom and CLDX’s CDX-0159, plus others.
TECHNICALS – Lower Lows for Many Reasons
The daily XBI index closed at 76, far below the 50-day moving average (MA) of 88 and easily breaking through the critical 80-81 support level. If there’s no change in the pillars driving the world’s risk these days – war (Russia/Ukraine), inflation (gas and all other prices) and even COVID (spiking domestically and worse overseas), interest rates (largest spike in recent history) – and technically, the biotechs are likely to be for sale. With all the forces slowing global growth, the “r” word (recession) is peaking its ugly head. Between mid-March and very early April, the XBI was above the 50-day MA but the inertia is painfully negative. The release of ASCO abstract titles (4/27) has done nothing and we’ll see how the group trades as we get closer to the actual event when the data is more mature and real time than the abstracts suggest. The Russia/Ukraine crisis is not ending any day soon (Pres. Biden just asked for $33 billion to support Ukraine through September). With prices and costs rising, the Fed’s only tool is rising rates and tapering – inflation is out of control. But that may actually accelerate the current slowdown and again resulting in a recession. Every time we think most of that of this bad news is discounted into the XBI, we rub our eyes when we see the unrelenting selloffs in the sector. No one thinks Powell can navigate a soft landing, especially with oil prices flying so high – but any change in the 4-5 risk factors listed above should lead to a jump in the bios, too. But the damage has certainly been done. The XBI chart is just horrible and the RSI of 33 is sitting barely above oversold levels, down significantly from 46 in the last Issue. The daily MACD has is still on the way down. Believe it or not, there is some good news out there (premium takeovers, better sales/earnings, FDA approvals and corporate deals) but no one wants to own very risky biotech stocks right now. It will change someday but that’s also like calling the end of the Ukraine crisis, or when rates will stop going up.
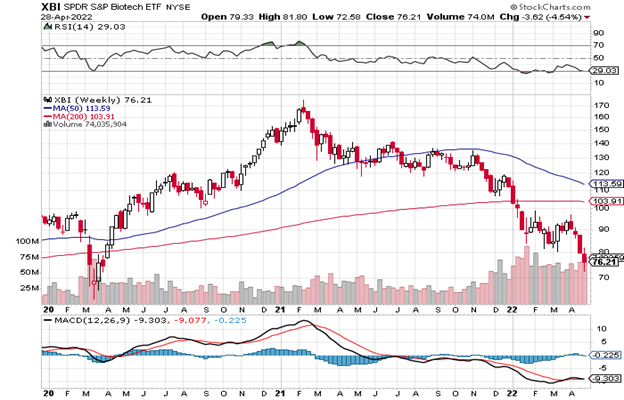
The weekly XBI is back to oversold levels with the RSI at 29, down from 36 at the last Issue. 104 is the next resistance number for the 200-week MA but we only see rallies that are often sold until the charts stabilize. Quarterly earnings at bios are separating some winners from losers but the small and microcaps are just too many and too tiny to matter today. Investors will demand a lot – ironically even at these levels. One small iota of interest is that some venture capitalists (VCs) are buying public stocks at current levels. Stay with well-financed, quality managed companies and, for those with binary events coming up the market is likely to wait for the news and buy the quality data after the fact.
Upcoming Earnings Calls for MTSL Universe:
| STOCK | DATE | TIME (in ET) |
| ESPR | 5/3 | 8:00am |
| INCY | 5/3 | 8:00am |
| PCRX | 5/4 | 8:30am |
| IONS | 5/4 | 11:30am |
| ACAD | 5/4 | 4:30pm |
| SGMO | 5/5 | 4:30pm |
| MDGL | 5/9 | 8:00am |
| MYOV | 5/10 | 8:30am |
| PLXP | 5/13 | 8:30am |
Clinical Trials Watch
Company Updates
ALKS — Q1 Excels – Lybalvi Strong Out of the Gate & Existing Strength Across The Board
Lybalvi is off to a strong start and has exceeded Wall Street’s expectations with $13.9 miilion in revenue vs. the $9 million consensus estimate for the drug’s first full quarter since launch. The stronger than expected Q1;22 results for LYBALVI led a solid first full quarter for ALKS with total revenues of $278.5 million easily beating the Wall Street consensus of $256 million. Outside of Lybalvi, Vivitrol came in at $84.9 million vs. the Wall Street consensus of $80 million, and Aristada revenue was $72.5 million vs. the Wall Street consensus of $67 million. Higher manufacturing and royalty revenues from Vumerity also drove the beat.
In our view, Lybalvi’s has peak sales potential of over a $1 billion based on initial demand trends with clinician receptivity to the product’s unique profile (significantly less weight gain vs. Olanzapine) and positioning. We are encouraged by both the quarterly results and 2022 outlook and by management commentary on launch dynamics to date. The extent of Olanzapine switching (representing 50% of Lybalvi patients to date), and also the extent of patient capture in the Bipolar indication (50% of patients) given the larger market opportunity in bipolar disease vs. schizophrenia are both positives for the drug’s launch. While Olanzapine has historically seen greater use in this indication, the bipolar patient population is potentially more attractive from a commercial perspective, given that this group is more heavily weighted toward patients with commercial payer coverage (i.e., potential for higher net price).
In April 2022, ALKS began arbitration proceedings related to Janssen’s partial termination of two license agreements with the company in the U.S. and Janssen’s royalty and other obligations under the agreements. Under these agreements, Janssen received access and rights to Alkermes‘ small particle pharmaceutical compound technology, known as NanoCrystal® Technology, which enabled the development and commercialization of a number of successful products, such as INVEGA SUSTENNA, INVEGA TRINZA, INVEGA HAFYERA and CABENUVA®.
Janssen partially terminated these agreements in the United States effective as of February 2022. In our view, the strong Lybalvi launch makes this less important and most of Wall Street has already discounted the royalty stream.
ALKS’ management has revisited raising guidance given the still relative early stages of the drug’s launch. However, in our view this is an opportunity and attractive entry point for new investors that are looking for strong drug launches. One more strong quarter would surely drive a string of upward forecasts from Wall Street and drive the stock higher. In our view, Lybalvi’s has peak sales potential of over a $1 billion based on initial demand trends with clinician receptivity to the product’s unique profile (significantly less weight gain vs. Olanzapine) and positioning. As a reminder, ALKS is a major investor of activist superstar Alex Denner’s Sarissa Capital, who now owns 10% of the stock. Whatever changes ALKS has undergone since the Sarissa involvement, they are working.
BMRN — BMRN’S Voxzogo Launch Strong, Guidance Raised, Roctavian Approvals in 2022
BMRN recently reported their financials for Q1 and delivered revenue of $519 million (+7% Y/Y) which was slightly below the Wall Street consensus estimate $509 million. In our view, investor focus this year continues to be on the Voxzogo’s launch, which has been so strong out of the gate that the company has already raised guidance to $100-125 million from $90-115 million. The potential EU and US Roctavian approvals in mid/3Q and 4Q, respectively will also continue to attract investor attention. Within the early gene therapy pipeline, Phase I/II dosing of BMN 331 (HAE) has begun while BMN 307 (PKU) will remain on clinical hold for several more quarters pending non-clinical study results.
Voxzogo is clearly exceeding expectations as 1Q net sales of $20 million easily toppced the Wall Street consensus estimate of $15 million, with 2/3 from ex-US regions. The drug is now commercial in 15 markets, with 284 children (83 US, 201 ex-US) on paid drug as of 3/31 and 53+ in process in US. Two important upcoming events for the drug include potential approval in the key Japan market in late 2022 and discussing potential expansion to <5 year olds with the FDA after recent positive Phase II data.
Two Roctavian reviews are upcoming with a CHMP opinion now expected mid-2022 (previously estimated 2Q), and a June BLA re-submission would imply potential US approval by year end. The company said that previously reported Phase I/II salivary gland mass data deemed not attributable to Roctavian will be presented next month at ASGCT and will also be provided to FDA/EMA as part of safety/regulatory reviews.
BMRN’s core business remains stable with solid Naglazyme (+19% Y/Y) and Vimizim (+16% Y/Y) sales on large ex-US order fulfillment, with robust Brineura growth (+33% Y/Y) reflecting new patient starts (+18%). Palynziq (+2% Y/Y) came in well below our expectations on seasonality and continued COVID-19 impact on clinics, but should gain more ground through 2022. Kuvan decline flattening out ($59M in 1Q). B
In our view, BMRN’s broadly reiterated strong product sales guidance for Voxzogo is a reflection of the management’s ability to launch/sell drugs. BMRN is a world’s leader in orphan drugs and is quite skilled at achieving regulatory approvals and quickly following up with strong product launches. In troubled times for development stage biotechs, one can appreciate the solid/steady orphan drug business that BMRN has built.
INCY — INCY Inks Deal With Maruho to Develop Ruxolitinib in Japan
INCY recently inked a deal with the Japanese specialty pharmaceutical company who focuses in dermatology, Maruho Co., Ltd., for the development, manufacturing and exclusive commercialization of ruxolitinib cream, a novel cream formulation of INCY’s selective JAK2 inhibitor ruxolitinib, for treatment of autoimmune and inflammatory dermatology indications in Japan. A topical formulation which some on Wall Street have modest expectations for, in our view, ruxolitinib has significant potential in previously underserved inflammatory dermatologic diseases, including vitiligo and atopic dermatitis.
Under the terms of the agreement, Maruho will make an upfront payment to INCY and will also receive additional potential development, regulatory and commercial milestones and royalties on net sales of the licensed product in Japan. In return, Maruho will receive the rights to develop, manufacture and exclusively commercialize ruxolitinib cream, and other potential future topical formulations of ruxolitinib, in autoimmune and inflammatory dermatologic diseases, including vitiligo and atopic dermatitis, in Japan.
Japan is a difficult pharmaceutical market to access without a partner. In addition, at least one clinical trial will likely be needed to achieve Japanese regulatory approval which Maruho will pay for and conduct. Maruho is an ideal partner as are a specialty pharmaceutical company in dermatology who will support the development of ruxolitinib cream in Japan, including both vitiligo and atopic dermatitis. Maruho has already stated that they are beginning with atopic dermatitis development as soon as possible. A topical formulation which some on Wall Street have modest expectations for, in our view, ruxolitinib has significant potential in previously underserved inflammatory dermatologic diseases, including vitiligo and atopic dermatitis.
The Back Page
| Symbol | Company | Orig.Rec. | Current | Target | Recommendation |
|---|---|---|---|---|---|
| ACAD | Acadia | 33.79 | 18.96 | 45 | BUY under $28 |
| ALKS | Alkermes | 10.13 | 29.79 | 55 | BUY under $35 |
| BCYC | Bicycle | 43.92 | 24.15 | 75 | BUY under $50 |
| BMRN | BioMarin | 12.68 | 82.64 | 150 | BUY under $100 |
| CLDX | Celldex | 10.50 | 31.22 | 85 | BUY under $55 |
| ESPR | Esperion | 24.42 | 5.51 | 25 | BUY under $10 |
| INCY | Incyte | 5.88 | 75.40 | 100 | BUY under $80 |
| IONS | Ionis | 7.63 | 39.55 | 65 | BUY under $50 |
| MDGL | Madrigal | 17.00 | 70.55 | 275 | BUY under $200 |
| MYOV | Myovant | 13.74 | 9.37 | 45 | BUY under $30 |
| PCRX | Pacira | 15.78 | 75.32 | 100 | BUY under $80 |
| PLXP | PLx Pharma | 16.70 | 3.22 | 35 | BUY under $22 |
| PGEN | Precigen | 34.42 | 1.40 | 24 | BUY under $12 |
| SGMO | Sangamo | 4.77 | 4.32 | 30 | BUY under $20 |
| TCRT | Alaunos | 8.00 | 0.43 | 12 | BUY under $5 |
| VXRT | Vaxart | 8.00 | 3.69 | 30 | BUY under $15 |
| ZYNE | Zynerba | 8.00 | 1.40 | 16 | BUY under $8 |
*New recommendation.
THE MODEL PORTFOLIO*
| COMPANY | SHARES OWNED | TOTAL COST | TODAY’S VALUE |
|---|---|---|---|
| Long Positions | |||
| Acadia | 4,750 | 156,557 | 90,060 |
| Alaunos | 26,125 | 166,100 | 113,202 |
| Alkermes | 3,800 | 88,690 | 494,275 |
| Bicycle | 2,400 | 105,408 | 57,960 |
| Celldex | 15,832 | 174,993 | 494,275 |
| Esperion | 3,316 | 105,316 | 18,271 |
| Incyte | 1,229 | 34,817 | 92,667 |
| Ionis | 3,087 | 49,123 | 122,091 |
| Madrigal | 3,127 | 69,980 | 220,610 |
| Myovant | 7,125 | 103,853 | 66,761 |
| Pacira | 2,375 | 63,887 | 178,885 |
| PLx Pharma | 15,359 | 269,988 | 49,456 |
| Precigen | 9,690 | 76,510 | 13,566 |
| Sangamo | 19,456 | 253,596 | 84,050 |
| Vaxart | 29,687 | 250,000 | 109,545 |
| Zynerba | 10,192 | 150,003 | 14,270 |
| (04/28/22) | Equities: | $1,736,994 | |
| Cash: | $27,796 | ||
| PORTFOLIO VALUE: | $1,764,790 |
*The Model Portfolio is designed to reflect specific recommendations. We began the Model Portfolio on 12/23/83 with $100,000. On 4/13/84, we became fully invested. All profits are reinvested. Stocks recommended since then may be equally attractive, but may not be in the Model Portfolio. Transactions and positions are valued at closing prices. No dividends are created, and we don’t use margin. Interest income is credited only on large cash balances.
The Model Portfolio
THE TRADER’S PORTFOLIO**
| COMPANY | SHARES OWNED | TOTAL COST | TODAY’S VALUE |
|---|---|---|---|
| Long Positions | |||
| Acadia | 4,750 | 156,557 | 90,060 |
| Alaunos | 26,125 | 166,100 | 11,325 |
| Alkermes | 3,325 | 83,184 | 99,052 |
| Bicycle | 2,554 | 107,779 | 61,679 |
| Celldex | 15,832 | 174,993 | 494,275 |
| Esperion | 3,871 | 100,005 | 21,329 |
| Incyte | 2,117 | 51,176 | 159,622 |
| Ionis | 3,135 | 53,501 | 123,989 |
| Madrigal | 2,764 | 49,964 | 195,000 |
| Myovant | 7,039 | 102,831 | 65,955 |
| Pacira | 1,900 | 55,918 | 143,108 |
| PLx Pharma | 15,358 | 269,988 | 49,453 |
| Precigen | 9,641 | 119,952 | 13,497 |
| Sangamo | 19,455 | 253,596 | 84,046 |
| Vaxart | 29,687 | 250,000 | 109,545 |
| Zynerba | 6,795 | 99,997 | 9,513 |
| (04/28/22) | Position Total: | $1,731,449 | |
| Margin: | -$1,256,899 | ||
| PORTFOLIO VALUE: | $474,549 |
**The Trader’s Portfolio joined the Model Portfolio on 1/6/05 with $500,000 and is designed to take advantage of short-term opportunities throughout the biotech sector. The Trader’s Portfolio will hold both long and short positions in stocks, trade-in options, and use margin. These strategies increase risk. Although there is no limit on the time any purchase can be held, the time frame for most investments will be weeks to months.
The Trader’s Portfolio
BENCHMARKS
| NASDAQ | S&P 500 | MODEL | TRADER‘S | |
|---|---|---|---|---|
| Last 2 Weeks | -3.5% | -2.4% | -10.4% |
-30.4% |
| 2022 YTD | -17.7% | -10.1% | -22.5% | -51.3% |
| Calendar Year 2021 | 21.3% | 26.9% | -15.2% | -29.5% |
| Calendar Year 2020 | 43.6% | 16.3% | 13.8% | 29.1% |
| Calendar Year 2019 | 35.2% | 28.8% | 10.7% | 44.1% |
| Calendar Year 2018 | 5.7% | 6.6% | 4.5% | 11.2% |
| Calendar Year 2017 | 29.3% | 19.9% | 65.6% | 98.9% |
| Calendar Year 2016 | 7.5% | 9.5% | -29.6% | -30.5% |
| Calendar Year 2015 | -0.1% | -0.1% | 25.1% | 27.9% |
| Calendar Year 2014 | 13.4% | 11.4% | 29.2% | 45.0% |
| Calendar Year 2013 | 38.3% | 29.6% | 103.4% | 214.7% |
BENCHMARKS
New Money Buys
NEW MONEY BUYS
Contact Info
Medical Technology Stock Letter
John McCamant, Editor
Jay Silverman, Editor
Jim McCamant, Editor-at-Large
Joan Wallner, Associate
BioInvest.com
579 Mangels Ave.
San Francisco, CA 94127
510-843-1857
Send us an email
Download a PDF of MTSL Issue #975
©Piedmont Venture Group (2022). Address: P.O. Box 40460, Berkeley, CA 94706. Telephone: (510) 843-1857. Fax: (510) 843-0901. BioInvest.com. Email: admin@bioinvest.com. Published 24 times a year. Email subscription rates: 1 year – $399; 2 years – $678; 3 years – $898. You may cancel at any time for a prorated refund. The information and opinions contained herein have been compiled or arrived at from sources believed to be reliable but no representations or warranty, express or implied, is made as to the accuracy or completeness. In no way shall this newsletter be construed as an offer to sell or solicitation of an offer to buy any securities. The publisher and its associates, directors or employees may have positions in, and may from time to time make purchases or sales of, securities mentioned herein. We cannot guarantee and you should not assume that future recommendations will equal the performance of past recommendations or be profitable.