MTSL Issue 882
IN THIS ISSUE: CRSPR – The Tip of the Titanic Iceberg?
Since Last Issue: BTK: 5.6%; NBI: 5.6%; Model Portfolio: -1.5%; Trader’s Portfolio: -1.6%
SENTIMENT – It’s Summertime, Enjoy The Quiet
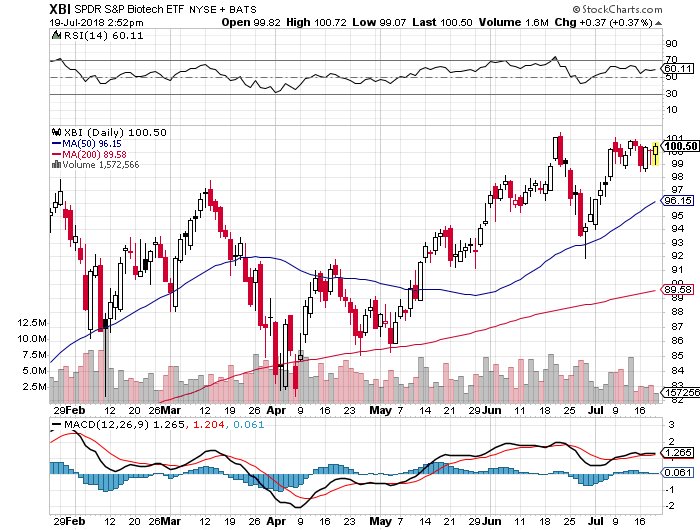
One of the most refreshing and needed things during vacation is to relax. That just isn’t the case in biotech with daily, stock-moving headlines in all factions of the industry (e.g., regulatory, scientific, corporate, finance, etc.), regardless of the time of the year. As we approach second quarter earnings calls, company updates will be analyzed with fine-tooth combs but the overall message is that the sector is still rather healthy. Positive fund flows remain in place and the Big Boys of biotech have caught up with the smaller, riskier babies. In fact, the larger-cap weighted IBB has handily outperformed the XBI index since the beginning of July. Led by BIIB’s Alzheimer’s surprise but also VRTX™s indirect fortune, the big money resides in the big names.
The XBI has not been able to break above the mid-June high, yet still resides well above the 50-day moving average and also the 200-day MA. After an impressive recovery from the end of June selloff, the RSI and MACD are in a neutral/slightly overbought position. As we mentioned in the last Issue, the overflowing cash levels of the global Big Boys of Drug/Biotechland lead us to believe that renewed M&A activity is nearing.
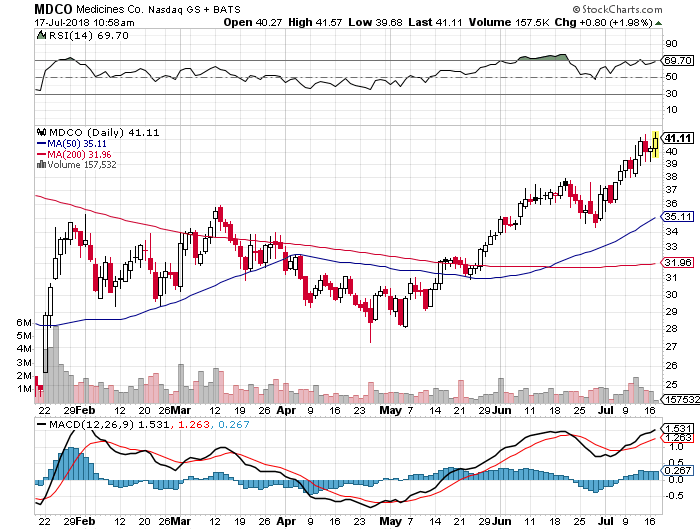
That takeover theory brings us to one MTSL recommended stock that is steadily moving higher without much news: The Medicines Company (MDCO). While the gene therapy noise continues to only get louder (see CRSPR comments below), MDCO has been relatively quiet. Although they recently reported an impressive third safety and enrollment update on the inclisiran ORION trials, the Company has not announced any major new clinical data and most know that their Phase III trials are not due to readout until about a year from now. With activist Denner at the Board’s helm, we still believe it is just a matter of time that MDCO“ with inclisiran’s light shining even brighter – becomes a bona fide M&A target.
Second quarter earnings are on the docket starting this week for the entire biotech/drug group, so enjoy the quiet while you can, for in biotech it usually doesn’t last very long.
DATA – CELG – The Ball Keeps Rolling
More good news as Celgene released positive results from a late-stage trial of a treatment for breast cancer as the trial met its co-primary endpoint of progression-free survival. The trial, which was sponsored by Roche, involved 902 patients and sought to evaluate the combination of the investigational combination Tecentriq plus CELG’s Abraxane in triple negative breast cancer, a type that has a high unmet need. This is the first Phase III study to demonstrate a statistically significant PFS improvement in first-line metastatic or unresectable locally advanced triple negative breast cancer (TNBC). The results will be presented at an upcoming medical meeting.
REGULATORY/RESEARCH
FDA Issues Gene Therapy Guidelines/NATURE Article Slams CRISPR ODELs Positive For SMGO, Negative For CRISPR – BUY SGMO and SHORT CRSP, EDIT, NTLA
On July 12, the FDA released the highly-anticipated gene therapy guidance documents. After reviewing the draft guidelines, while the FDA is no doubt optimistic of the future of gene therapy, in our view, the proposals suggest that there is a lot to prove before a Company even begins human gene editing studies. Right after the FDA guidelines were released, Nature Biotechnology published what we believe is the most negative, objective research study on CRSPR to date, calling into question the viability of that technology as a therapeutic option in the near-term. As a result, we are reiterating our BUY recommendation on SGMO. We are also initiating SHORT SALE recommendations on three CRISPR-related gene-editing stocks – CRSP, EDIT and NTLA.
The commercial markets for gene therapy are obviously large and the possible cures no doubt evolutionary. According to the FDA documents, “the National Institutes of Health (NIH) reports that nearly 7,000 rare diseases affect more than 25 million Americans. Approximately 80% of rare diseases are caused by a single-gene defect, and about half of all rare diseases affect children. Since most rare diseases have no approved therapies, there is a significant unmet need for effective treatments, and many rare diseases are serious or life-threatening conditions. As a general matter, developing safe and effective products to treat rare diseases can be challenging. For example, it might be more difficult to find and recruit patients with rare diseases into clinical trials. Additionally, many rare diseases exhibit a number of variations or sub-types. Consequently, patients may have highly diverse clinical manifestations and rates of disease progression with unpredictable clinical courses. These challenges are also present for the development of gene therapy (GT) products. However, despite these challenges, GT-related research and development in the area of rare diseases continues to grow at a rapid rate.
CMC Requirements for Gene Therapy and Gene Editing Are Demanding
The IND process is by no means simple. One should contain items typical for a conventional biopharmaceutical product, but the manufacturing process in gene therapy/editing (GT) takes precedent. The process and control information must include proper nomenclature, molecular structure including genetic sequence, relevant regulatory elements (promoters/enhancers, introns, poly(A) signals), restriction enzyme sites, functional components, as well as cellular components of the drug product (https://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/CellularandGeneTherapy/UCM610795.pdf).
For viral vectors, a description of the composition of the viral capsid, envelope structures, available modifications shall be included, as well as biophysical characteristics including molecular weight, particle size, and biochemical characteristics (e.g. glycosylation sites). The nature of the genome of viral vectors, whether single stranded, double stranded, or self-complementary, DNA or RNA and copy number of genomes per particle should be included. For bacterial vectors, physical and biochemical properties, growth characteristics, genetic markers (e.g. auxotrophic or attenuating mutations, antibiotic resistance) and the location (e.g. on the plasmid, episome, or chromosome), as well as description of any inserted foreign genes and regulatory elements should be included. For ex-vivo genetically modified cells, the major and minor cell populations, as well as the vector that contains the transgene cassette that is transferred into the cell shall be described. For cells that have been genetically modified using genome editing, the nature and method of genomic modifications should be included. Moreover, potential off-target effects should be noted in the preclinical package. This last item, in our view, among other challenges, may be the single largest risk factor for CRSPR products specifically (e.g., the FDA placed a clinical hold on CRSP before the start of its first human trial).
Long-term Monitoring for Gene Therapy and Gene Editing – 15 Years
After reviewing the documents, in our view the greatest challenge is the current unknown result of editing genes – off-target toxicities that may occur long after the initial single gene insertion or deletion. Unexpected breaks in double stranded DNAs could either turn on/off genes with various permanent consequences. The objective of long-term follow-up observations is to “to capture delayed adverse events in subjects as well as to understand the persistence of the gene therapy product. In the new guidelines, the FDA recommends carefully selecting the patient population and disease to be treated when designing such protocols given patients with short life expectancies, multiple co-morbidities, and exposure to other agents such as radiation or chemotherapy could confound results. Further considerations include the duration of the long-term follow-up period, which the FDA recommends 15 years for integrating vectors such as gammaretroviral and lentiviral vectors and transposon elements, up to 15 years for genome editing products, and up to five years for AAV vector products. Importantly, the FDA recommends testing subjects at least annually for persistent vector sequences until they become undetectable. It is recommended that the sponsor “sample the likely population of transduced cells without being overly invasive (e.g., peripheral blood is suitable to test for the presence of hematopoietic stem cells rather than bone marrow biopsy).
In the context of the gene-editing guidelines, it is recommended that the sponsor propose a long-term follow up plan that covers monitoring of potential delayed adverse events that may result from off-target activities. In other words, in vivo/in vitro analysis such as INDEL – insertion and deletion of bases in a genome, as well as any adverse effects that may arise from the specific organ system the gene editing system targets. This again, to us, is a major potential roadblock for companies that use CRISPR as their primary gene editing tool and is probably why they have yet to be cleared for human development by the FDA. On the flipside, it supports the multiple INDs that have been granted to Sangamo (and BioMarin which is gene therapy and not gene editing) over the past two years with more to come, and the likely fact that the Company’s ZFNs – which are not CRISPR technology – result in undetectable off-target effects utilizing the most sensitive assays around.
More Bad News for CRISPR Stocks as Nature Publishes More Safety Issues Concerning Off-Target, Unintended Insertions/Deletions
Research published this past Monday in the prestigious scientific journal NATURE BIOTECHNOLOGY suggests that the FDA’s concerns with off-target INDELs is only the tip of a Titanic-sized iceberg for CRISPR-focused stocks – CRISPR-Cas9 can cause significantly greater genetic havoc than experts previously thought, and the study concludes perhaps enough to threaten the health of patients who would one day receive CRISPR-based therapy. The DNA damage found in the new study included deletions of thousands of DNA bases, including at spots far from the edit site. Some of the deletions can silence genes that should be active and activate genes that should be silent, including cancer-causing genes. The DNA chaos that CRISPR unleashes has been “seriously underestimated,” said geneticist Allan Bradley of England’s Wellcome Sanger Center, who led the study. “This should be a wake-up call.”
This leads to the question of why haven’t scientists from the CRISPR companies seen off-target effects when they analyze the DNA of CRISPR’d cells “You find what you look for,” said Bradley. “No one is looking at the impact (of these DNA changes) on downstream genes.” And few studies conduct full-out genome sequencing of CRISPR’d cells. Moreover, scientists typically search for one form of the collateral damage the Sanger study found – deletions of thousands of DNA bases (the double helix’s famous A’s, T’s, C’s, and G’s) – using a standard PCR, which makes millions of DNA copies. But to work, PCR must attach to a “binding site” on DNA; CRISPR sometimes deletes that binding site, and Bradley’s team used a different technique to analyze the double helix for collateral damage from CRISPR.
The Sanger scientists did not set out to find collateral DNA damage from CRISPR. As they investigated how CRISPR might change gene expression, a “weird thing” showed up – the target DNA was accurately changed, but that set off a chain reaction that engulfed genes far from the target. The scientists therefore changed course. When they aimed CRISPR at different targets in mouse embryonic stem cells, mouse blood-making cells, and human retinal cells, “extensive on-target genomic damage [was] a common outcome. In one case, genomes in about two-thirds of the CRISPR’d cells showed the expected small-scale inadvertent havoc, but 21% had DNA deletions of more than 250 bases and up to 6,000 bases long.
After learning of these results, Nature Biotechnology took a year to publish the paper, after asking Bradley numerous variations of “are you sure?” and “did you consider this?” and asking him to run additional experiments. The results all held up. Gaetan Burgio, a CRISPR expert at Australian National University, pointed out in a series of tweets that “we all assumed the on-target specificity of Cas9,” meaning that if it hit its intended target, as it did in the Sanger experiments, there would be no far-flung genomic havoc. The new study, just published in Nature Biotechnology, is the first to actually test that assumption, but “others are under peer review [at] publications and will confirm the results presented in this paper.” With more negative CRISPR articles already undergoing peer-review, negative scientific publicity for CRISPR will continue (of course, it is expected there will be positive CRSPR studies done, too, but we believe the data will continue to weigh the risk/rewards). And this is our near-term conclusion with CRSP, NTLA and EDIT – once they or others begin to look for IDELs and off-target toxicities using even higher sensitive assays at the start and then over time, we believe the odds are growing that they too will find the same negative results as Bradley did.
To our knowledge, there are currently no bearish/Sell recommendations on the Street that have research coverage of CRSP, EDIT and NTLA. Theirs – and the respective companies – response to these negative studies are all the same – they use a different CRISPR than we do … we are aware of this situation and continue to monitor these issues … we haven’t seen this in our data or from analysts, they will eventually figure it out – and continue to recommend buying on weakness. Some Wall Street opinions are also likely due to investment banking relationships.
We believe that (a) the recent, unbiased published CRISPR research is extremely concerning (with more to come); (b) regulatory events such as INDs granted by some (ZFNs/gene therapy) and held up by others will continue to emphasize the agency’s caution towards CRISPR as a class; and (c) soon-to-be released clinical data will begin to and will further differentiate the haves (SGMO) and have nots (CRSP, EDIT, NTLA) in the gene editing space and consequentially shrink the massive discrepancy in valuations between the two. SGMO often gets caught up in the negative CRSPR press, we believe due to either unsophisticated and/or lazy reporters who cannot or do not care to distinguish the fact that SGMO’s ZFNs are not CRISPR-based and more importantly, that the Company has had no problems getting INDs granted and clinical trials approved and initiated.
Upcoming Catalysts in Gene Editing Stocks
By late summer, SGMO will release the first in human gene editing safety and efficacy data in MPSII or Hunter Syndrome (and also gene therapy initial results in hemophilia A with partner PFE). Either should serve as a significant positive catalyst for SGMO (and we believe a perceived negative for CRSP, EDIT, NTLA). Other significant pessimistic events for the CRISPR stocks will be the publication in peer-reviewed journals that further support the severe safety concerns documented in this week’s Nature Biotechnology study. By delaying the Nature article for a full year, it is quite possible that the next articles will be published relatively quickly, leading to a rapid-fire onslaught of negative publicity and possible further regulatory delays. The fact is that other studies have already been completed and only need to complete the peer review process before being released. Lastly, Wall Street may not sleep forever on the CRISPR safety concerns and eventually an analyst will forego some banking fees and downgrade the CRISPR companies to what they are exciting, research-stage biotechs that have zero products in human clinical development and face increasing regulatory and scientific roadblocks along the way. The CRISPR IP landscape is also a complete mess with multiple competing claims (another one emerged this week), which in our view means that no company will dominate the field legally. SGMO, in stark contrast, has one of the most dominant IP estates in biotech with a complete lock on the zinc finger proteins.
The bottom line with gene editing is that once a gene is inserted, there is no turning back as the proverbial ‘genie’ has already been let out of the bottle. We know that CRSP’s first clinical trial has been put on hold by the FDA (May 31) and the Company publicly still expects to be given the agency’s blessing to begin the study by the end of the year. Their next quarterly report should provide the latest update on this issue. It would not be surprising to us to see, after the increasing load of published scary data, that the agency takes a even more cautious approach to CRISPR technology than it already has, which would lead to a continued major overhang for CRSP and likely the other CRISPR stocks and result in significant devaluations over time. And that realization, we also believe, will increase SGMO’s market value – relatively and absolutely.
We are recommending a SHORT SALE of all three CRISPR stocks and reiterating our STRONG BUY on SGMO.
- CRSP is a SHORT SALE up to 65 with a TARGET PRICE of 40
- EDIT is a SHORT SALE up to 40 with a TARGET PRICE of 26
- NTLA is a SHORT SALE up to 34 with a TARGET PRICE of 21
- SGMO is a BUY under 30 with a TARGET PRICE of 40
FDA Real Time Oncology Review
The FDA is speeding up approval times for new drugs, in particular cancer treatments. The agency recently approved NVS Kisqali (ribociclib) in combination with an aromatase inhibitor for the treatment of pre/perimenopausal or postmenopausal women with hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative advanced or metastatic breast cancer, as initial endocrine-based therapy. The FDA also approved Kisqali in combination with fulvestrant for the treatment of postmenopausal women with HR-positive, HER2-negative advanced or metastatic breast cancer, as initial endocrine based therapy or following disease progression on endocrine therapy. This is the first FDA approval granted under two new pilot programs announced earlier this year that collectively aim to make the development and review of cancer drugs more efficient. The FDA used these new approaches to allow the review team to start analyzing data before the actual submission of the application and help guide the sponsor’s analysis of the top-line data to tease out the most relevant information. This enabled formal approval in less than one month after the June 28 submission date and several months ahead of the goal date. (https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm613801.htm?utm_campaign=07182018_PR_FDA%20expands%20the%20use%20of%20breast%20cancer%20drug&utm_medium=email&utm_source=Eloqua).
IONS/AKCA Inotersen Approved In Europe; FDA Aggressively Pushing BioSimilars
AKCA and IONS announced that TEGSEDI (inotersen) received marketing authorization approval from the European Commission (EC) for the treatment of Stage 1 or Stage 2 polyneuropathy in adult patients with hereditary transthyretin amyloidosis (hATTR). So IONS/AKCA has beat ALNY’s patisiran to market in the EU, but is not expected to do so in the U.S. We have written about the competition of these two rare disease anti-sense drugs, where Alnylam appears to have the preferred compound. The two separate registration trials were not identical in design, but nonetheless the TEGSEDI approval gives IONS another commercialized compound.
FDA Attacks Biologics Patent Extensions By Aggressively Supporting Biosimilars
The FDA has issued a few new proposals for speeding up the approval of biosimilars. At a speech this week, Commissioner Gottlieb noted, while less than 2% of Americans use biologics, they represent 40% of total spending on prescription drugs. So, enabling a path to competition for biologics from biosimilars is a key to reducing costs and to facilitating more innovation. The new initiative, called the Biosimilars Action Plan, will target competition and affordability across the market for biologics and biosimilars (i.e., generic biologics). Biosimilars in many cases have failed to penetrate the brand named compounds. Furthermore, Big Bio/Big Pharma have astutely used very creative legal maneuvers to delay generic competition from entering the markets, despite receiving FDA approval. While not price controls, this act is another attempt by the agency/Trump administration to cut costs of expensive new medicines. Most past efforts have failed to do so, hence the jury is still out if the new policy will have any real impact.
As a result of the new short recommendations, we are making the following changes to the MTSL Portfolio:
Model Portfolio:
- Buy $200,000 of SGMO
- Sell Short $135,000 each of CRSP, EDIT & NTLA
- Sell $605,000 of MDGL (as it has vastly outperformed and now has too much weight in the portfolio)
Traders Portfolio:
- Buy $200,000 of SGMO
- Short Sell $135,000 of CRSP, EDIT, & NTLA
- Will use margin to cover $605,000 of transactions
Clinical Trials Watch
Company Updates
ESPR – Esperion Impresses on Investor Day
 We came away from Esperion’s Investor Day highly encouraged by the company’s detailed performance and particularly their professionalism. Bempedoic acid safety was addressed with positive physician comments from some of the world’s leading clinical researchers including Steve Nissen (Cleveland Clinic). Importantly, he viewed the recent fatality imbalance in one of the Phase III BA studies as “noise” with a lack of compelling scientific rationale and too few events to draw any conclusions, coupled with the point that the CLEAR long-term outcomes study, which he Chairs, remains ongoing after multiple DMC safety reviews. Dr. Nissen also pointed out that CLEAR should deliver better data than either of the Amgen or Regeneron PSCK9 MAb trials due to better trial design including a more suitable patient selection and longer treatment. Dr. John Jenkins (formerly of the FDA’s Office of New Drugs) echoed Dr. Nissen’s viewpoints regarding the safety of BA. Dr. Jenkins also provided a detailed presentation on the path for BA’s potential upcoming approval.
We came away from Esperion’s Investor Day highly encouraged by the company’s detailed performance and particularly their professionalism. Bempedoic acid safety was addressed with positive physician comments from some of the world’s leading clinical researchers including Steve Nissen (Cleveland Clinic). Importantly, he viewed the recent fatality imbalance in one of the Phase III BA studies as “noise” with a lack of compelling scientific rationale and too few events to draw any conclusions, coupled with the point that the CLEAR long-term outcomes study, which he Chairs, remains ongoing after multiple DMC safety reviews. Dr. Nissen also pointed out that CLEAR should deliver better data than either of the Amgen or Regeneron PSCK9 MAb trials due to better trial design including a more suitable patient selection and longer treatment. Dr. John Jenkins (formerly of the FDA’s Office of New Drugs) echoed Dr. Nissen’s viewpoints regarding the safety of BA. Dr. Jenkins also provided a detailed presentation on the path for BA’s potential upcoming approval.
In our view, ESPR did a thorough job of detailing the safety for the 4,000 treated patients which demonstrates the excellent safety profile of bempedoic acid. Lots of upcoming news. The next potential catalyst for ESPR is the BA/EZ combo data in August. This will be followed by BA data from Study 2 in late September. In addition pre-clinical NASH data will be published in a peer-reviewed journal, Phase 1 data on the sustained release formulation of BA, and Phase II plans for NASH will occur in Q4
MYOV – Completes Screening For LIBERTY 1
 MYOV announced that it has completed screening patients for its LIBERTY 1 study, the first of two Phase III replicate studies evaluating relugolix in women with heavy menstrual bleeding associated with uterine fibroids. The company expects to complete screening for LIBERTY 2 this quarter and report top-line efficacy and safety data for LIBERTY 1 in the second quarter of 2019.
MYOV announced that it has completed screening patients for its LIBERTY 1 study, the first of two Phase III replicate studies evaluating relugolix in women with heavy menstrual bleeding associated with uterine fibroids. The company expects to complete screening for LIBERTY 2 this quarter and report top-line efficacy and safety data for LIBERTY 1 in the second quarter of 2019.
About Myovant’s Phase III Program for Uterine Fibroids
Myovant is currently conducting a Phase III clinical program consisting of two international, replicate pivotal clinical trials (LIBERTY 1 and LIBERTY 2), initiated in January 2017, of relugolix in women with heavy menstrual bleeding associated with uterine fibroids. Women with heavy menstrual bleeding associated with uterine fibroids in the LIBERTY 1 and LIBERTY 2 trials undergo a screening period requiring up to two menstrual cycles to document heavy menstrual bleeding and are then randomized in a 1:1:1 ratio to one of three arms. Women receive treatment with relugolix 40 mg once daily co-administered with commercially available hormonal add-back therapy for 24 weeks, relugolix 40 mg once daily monotherapy for 12 weeks followed by relugolix 40 mg once daily co-administered with hormonal add-back therapy for an additional 12 weeks, or placebo once daily for a period of 24 weeks. Myovant expects to enroll approximately 390 women in each of the two replicate LIBERTY 1 and LIBERTY 2 trials, with 130 women in each of the two active treatment arms and 130 women in the placebo arm. To be enrolled, women must have a monthly menstrual blood loss of at least 80 mL, measured by the alkaline hematin method, a quantitative measure of menstrual blood loss.
The primary efficacy endpoint for LIBERTY 1 and LIBERTY 2 is the proportion of all women enrolled who achieve a menstrual blood loss volume of less than 80 mL and at least a 50 percent reduction in menstrual blood loss volume from baseline over the last month of treatment as measured by the alkaline hematin method. Secondary efficacy endpoints include measures of change from baseline in hemoglobin, assessment of the impact of therapy on quality-of-life measures, reduction in uterine and fibroid volume, and pain reduction. Safety, including bone mineral density changes as measured by dual-energy x-ray absorptiometry, is also being assessed.
Eligible women completing the LIBERTY 1 or LIBERTY 2 trial will be offered the opportunity to enroll in an active treatment extension study in which all patients will receive relugolix 40 mg once daily co-administered with hormonal add-back therapy for an additional 28-week period, for a total treatment period of 52 weeks, to evaluate the safety and sustained efficacy of longer-term treatment.
MYOV has done a good job of executing their Phase III development program for relugolix positioning the company to file for FDA approval in 2019. The company also recently raised cash (~$75 million) with the clear goal of using it to prepare for commercialization. We share management’s confidence in the approvability of relugolix.
PCRX – Q2 Pre-Announcement Shows EXPAREL Momentum Intact
 Pacira reported preliminary EXPAREL net product sales of $80.4 million for the second quarter of 2018, a gain of 15% percent from Q2:17. During the second quarter of 2018, average daily sales grew 11%, 16% and 18% for April, May, and June, respectively, compared with the prior year. The numbers are further evidence of the expanding adoption of EXPAREL as an integral component of multimodal, non-opioid pain management strategies in a variety of surgical procedures.
Pacira reported preliminary EXPAREL net product sales of $80.4 million for the second quarter of 2018, a gain of 15% percent from Q2:17. During the second quarter of 2018, average daily sales grew 11%, 16% and 18% for April, May, and June, respectively, compared with the prior year. The numbers are further evidence of the expanding adoption of EXPAREL as an integral component of multimodal, non-opioid pain management strategies in a variety of surgical procedures.
The DePuy/Johnson & Johnson partnership is proceeding quite well and the upcoming launch of EXPAREL as the first long-acting, single-dose nerve block for upper extremity surgeries is already seeing increasing demand from new and existing accounts. Pacira expects to report its complete financial results Q2:18, along with financial guidance for 2018, during the company’s earnings call scheduled in August 2018.
The Back Page
| Symbol | Company | Orig.Rec. | Current | Target | Recommendation |
|---|---|---|---|---|---|
| ACAD | Acadia | 33.79 | 17.08 | 45 | BUY under $32 |
| ALKS | Alkermes | 10.13 | 45.28 | 75 | BUY under $55 |
| BMRN | BioMarin | 12.68 | 102.87 | 130 | BUY under $100 |
| CELG | Celgene | 24.97 | 85.34 | 120 | BUY under $90 |
| ESPR | Esperion | 24.42 | 43.13 | 100 | BUY under $75 |
| FPRX | Five Prime | 16.29 | 17.16 | 45 | BUY under $30 |
| INCY | Incyte | 5.88 | 70.11 | 95 | BUY under $75 |
| XON | Intrexon | 34.42 | 14.67 | 45 | BUY under $25 |
| IONS | Ionis | 7.63 | 45.84 | 70 | BUY under $55 |
| MDGL | Madrigal | 17.00 | 288.72 | 400 | BUY under $300 |
| MDCO | Medicines Company | 31.98 | 40.08 | 65 | BUY under $40 |
| MYOV | Myovant | 13.74 | 20.30 | 25 | BUY under $17 |
| NKTR | Nektar | 4.66 | 49.08 | 120 | BUY under $95 |
| NVAX | Novavax | 2.44 | 1.42 | 4 | BUY under $2 |
| PCRX | Pacira | 15.78 | 37.00 | 50 | BUY under $35 |
| SGMO | Sangamo | 4.77 | 15.05 | 40 | BUY under $30 |
| Ziopharm | 8.00 | 3.04 | 18 | BUY under $12 | |
| CRSP* | Crispr | 58.39 | 58.39 | 40* | SELL under $64* |
| EDIT* | Editas | 36.13 | 36.13 | 26* | SELL under $40* |
| NTLA* | Intellia | 31.63 | 31.63 | 21* | SELL under $34* |
*new recommendation
THE MODEL PORTFOLIO*
| COMPANY | SHARES OWNED | TOTAL COST | TODAY’S VALUE |
|---|---|---|---|
| Long Positions | |||
| Acadia | 3,000 | 102,417 | 51,240 |
| Alkermes | 2,500 | 32,695 | 113,200 |
| Esperion | 3,491 | 105,316 | 150,567 |
| Five Prime | 3,250 | 91,136 | 55,770 |
| Incyte | 1,294 | 34,817 | 90,722 |
| Intrexon | 2,200 | 76,510 | 32,274 |
| Ionis | 3,250 | 49,123 | 148,980 |
| Madrigal* | 3,292* | 69,980* | 950,460* |
| Medicines Co | 2,600 | 19,380 | 104,208 |
| Myovant | 6,500 | 103,853 | 152,250 |
| Nektar | 6,500 | 63,277 | 319,020 |
| Novavax | 27,000 | 60,984 | 38,340 |
| Pacira | 1,500 | 23,907 | 55,500 |
| Sangamo* | 20,479* | 253,596* | 308,209* |
| 12,500 | 101,000 | 38,000 | |
| Short Positions | |||
| Crispr* | -2,315* | 134,998* | 134,998* |
| Editas* | -3,736* | 134,982* | 134,982* |
| Intellia* | -4,268* | 134,997* | 134,997* |
|
(07/19/18) |
Equities: | $2,203,770 | |
| Cash: | $821,484 | ||
| PORTFOLIO | VALUE: | $3,025,254 |
*The Model Portfolio is designed to reflect specific recommendations. We began the Model Portfolio on 12/23/83 with $100,000. On 4/13/84, we became fully invested. All profits are reinvested. Stocks recommended since then may be equally attractive, but may not be in the Model Portfolio. Transactions and positions are valued at closing prices. No dividends are created, and we don’t use margin. Interest income is credited only on large cash balances.
The Model Portfolio
THE TRADER’S PORTFOLIO**
| COMPANY | SHARES OWNED | TOTAL COST | TODAY’S VALUE |
|---|---|---|---|
| Long Positions | |||
| Acadia | 3,000 | 102,417 | 51,240 |
| Alkermes | 2,000 | 27,189 | 90,560 |
| Esperion | 4,075 | 100,005 | 175,755 |
| Five Prime | 4,020 | 70,679 | 68,983 |
| Incyte | 2,229 | 51,176 | 156,275 |
| Intrexon | 2,170 | 75,472 | 31,834 |
| Ionis | 3,300 | 53,501 | 151,272 |
| Madrigal* | 2,910* | 49,964* | 840,175* |
| Medicines Co | 1,250 | 40,375 | 50,100 |
| Myovant | 7,410 | 102,831 | 150,423 |
| Nektar | 6,000 | 36,411 | 294,480 |
| Novavax | 25,000 | 58,025 | 35,500 |
| Pacira | 1,000 | 15,938 | 37,000 |
| Sangamo* | 20,479* | 253,596* | 308,209* |
| 12,500 | 101,000 | 38,000 | |
| Short Positions | |||
| Crispr* | -2,315 | 134,988 | 134,988* |
| Editas* | -3,736 | 134,982 | 134,982* |
| Intellia* | -4,268 | 134,997 | 134,997* |
|
(07/19/18) |
Position Total: | $2,074,830 | |
| Margin: | –$485,818 | ||
| PORTFOLIO | VALUE: | $1,589,012 |
**The Trader’s Portfolio joined the Model Portfolio on 1/6/05 with $500,000 and is designed to take advantage of short-term opportunities throughout the biotech sector. The Trader’s Portfolio will hold both long and short positions in stocks, trade-in options, and use margin. These strategies increase risk. Although there is no limit on the time any purchase can be held, the time frame for most investments will be weeks to months.
The Model Portfolio
BENCHMARKS
| NASDAQ | S&P 500 | MODEL | TRADER‘S | |
|---|---|---|---|---|
| Last 2 Weeks | 3.1% | 2.5% | -1.5% |
-1.6% |
| 2018 YTD | 13.3% | 4.9% | 47.4% | 40.7% |
| Calendar Year 2017 | 29.3% | 19.9% | 65.6% | 98.9% |
| Calendar Year 2016 | 7.5% | 9.5% | -29.6% | -30.5% |
| Calendar Year 2015 | -0.1% | -0.1% | 25.1% | 27.9% |
| Calendar Year 2014 | 13.4% | 11.4% | 29.2% | 45.0% |
| Calendar Year 2013 | 38.3% | 29.6% | 103.4% | 214.7% |
| Calendar Year 2012 | 13.4% | 15.9% | 25.7% | 68.7% |
BENCHMARKS
New Money Buys
NEW MONEY BUYS
Contact Info
Medical Technology Stock Letter
John McCamant, Editor
Jay Silverman, Editor
Jim McCamant, Editor-at-Large
©Piedmont Venture Group (2018). Address: P.O. Box 40460, Berkeley, CA 94706. Telephone: (510) 843-1857. Fax: (510) 843-0901. BioInvest.com. Email: admin@bioinvest.com. Published 24 times a year. Email subscription rates: 1 year – $399; 2 years – $678; 3 years – $898. You may cancel at any time for a prorated refund. The information and opinions contained herein have been compiled or arrived at from sources believed to be reliable but no representations or warranty, express or implied, is made as to the accuracy or completeness. In no way shall this newsletter be construed as an offer to sell or solicitation of an offer to buy any securities. The publisher and its associates, directors or employees may have positions in, and may from time to time make purchases or sales of, securities mentioned herein. We cannot guarantee and you should not assume that future recommendations will equal the performance of past recommendations or be profitable.

Do you have any updated thoughts about ZIOP that would be relevant to share with subscribers? Indeed, valuation appears compelling-making one wonder if there is some bad news underlying current decline in market cap.