MTSL Issue 1003
IN THIS ISSUE: FDA Approves BIIB/Eisai’s Alzheimer’s Drug
Since Last Issue: BTK: 2.41%; NBI: 2.30%; XBI: 5.19%; Model Portfolio: 3.46%
SENTIMENT — Biotech Continues to Climb
FDA approves BIIB/Eisai’s Leqembi in early Alzheimer’s disease (AD)
The FDA has approved BIIB/Eisai’s Leqembi in early Alzheimer’s disease (AD). Overall, we remain positive on BIIB’s opportunity to address the early AD market which is expected to be huge. Wall Street estimates for worldwide Leqembi sales are as high as $15 billion. Importantly, the recent Centers for Medicare & Medicaid Services (CMS) announcement that Medicare will provide broad coverage for Leqembi with participation in a registry upon full FDA approval means a fast rollout as Medicare covers millions of patients. The full FDA approval is a monster win for both AD patients and BIIB/Easai.
Obesity Wars Heat Up As Lilly Starts Head-to-Head Trial Comparing Mounjaro to Wegovy
The obesity wars are heating up as Eli Lilly recently registered a Phase IIIb clinical trial, SURMOUNT-5, which compares Lilly’s Mounjaro with Novo’s Wegovy in obese or overweight patients with weight-related health conditions. The head-to-head Phase III Trial will enroll 700 participants from 61 sites across the U.S., Canada, South America and several European countries. It began in April and Lilly expects its completion around January 2025. The obesity market is huge and impacts 650 million people worldwide and nearly half of Americans. It is a chronic disease with limited treatment options that increases the risk of other weight-related conditions, such as diabetes and heart disease and affects overall health.
Huge Market: The tremendous unmet need for weight loss has resulted in staggering sales projections. Here is a smattering of the Wall Street’s best guesstimates; UBS projected that Mounjaro could reach peak sales of $25 billion, primarily in treating diabetes and obesity, TD Cowen analysts suggested that the global obesity drug market could reach $30 billion by 2030, with Novo and Lilly leading the charge. Morgan Stanley has the highest estimate at $50 billion.
Two Targets is Better Than One
Wegovy (semaglutide) stimulates the hormone glucagon-like peptide 1 (GLP-1) alone, while Mounjaro (tirzepatide) stimulates GLP-1 and glucose-dependent insulinotropic polypeptide (GIP). By targeting two receptors, Lilly’s drug stands to have a more significant effect. GIP and GLP-1 are hormones the gut produces in response to the nutrients in food. GIP and GLP-1 have diverse roles in the body and are responsible for the incretin effect. In healthy humans, GIP is responsible for nearly two-thirds of the incretin effect, generating a more significant impact on insulin secretion than GLP-1. GIP is also responsible for regulating body weight through appetite and food intake.
The new way of thinking about obesity is how these medications affect the body’s metabolic set point, not the appetite. Unlike metabolism, which can go up or down depending on diet, exercise and hormonal factors, the metabolic set rate remains fixed. Generally, this is why short-term diets fail—they don’t alter the set point. However, GLP-1 agonists can. The belief is that drugs that lower the set point, natural hunger will come down as a secondary byproduct of the drug. Lowering the metabolic set point also results in improvements in insulin resistance and how the body processes glucose.
VKTX-2735 Has Best in Class Potential
The recent Phase I data for ‘2735 was very good with promising tolerability with no discontinuations and low vomiting at the high dose. In our view, the excellent safety profile combined with very good weight loss mean that ‘2735 has best in class potential. A Phase II trial is expected to start soon and the company plans to implement a 3-week titration period in this trial compared to a more commonly used 4-week titration given the encouraging tolerability profile. The study will include four dosing arms with 15mg max dose in 100-150 patients. We also expect oral ‘2735 Phase I data by year-end. KOLs have recently pointed out that oral drugs with favorable tolerability should have a big advantage over injectable obesity drugs.
ASCO Review: NATELEE Provides Promise in Early Breast Cancer
The most important update at ASCO 2023 for early breast cancer patients came from the CDK4/6 inhibitor (CDK4/6i) class, Novartis’ Phase III NATALEE study with Kisqali (ribociclib) + ET for patients with early breast cancer. The data showed that adding Kisqali to a hormonal therapy reduced patients’ risk for recurrence by 25% compared to those on the endocrine therapy alone. Kisqali is currently approved to treat advanced and metastatic breast cancer and Novartis intends to submit for approval by the end of 2023. The results from the NATALEE trial of Kisqali in adjuvant HR+/HER2- early breast cancer will likely be practice-changing.
NATELEE is very important as that there is an ongoing shift in the breast cancer community to Novartis’ ribociclib, which we think could carry subsequent implications for companies developing combination strategies with CDK4/6i. The data showed that the 3-year invasive disease free survival (iDFS) rate with ribociclib + non-steroidal aromatase inhibitor (NSAI) was 90.4% versus 87.1% in patients receiving NSAI alone (p=0.0014, HR=0.748). The ribociclib + NSAI combination arm reduced the risk of invasive disease by 25.2% versus NSAI alone. Novartis plans to conduct additional follow-up in regard to OS. The ability to treat breast cancer earlier can be a game changer. Novartis is also looking to replace adjuvant chemo in early breast cancer with Kisqali and that Phase III trial will read out in 2025.
FEAR & GREED – Extreme Greed
The F&G Index is now in Extreme Greed at 77 after closing at 67 two weeks ago. The index has continued to move higher for the last month with the most recent strength into Extreme Greed coming after the Debt Deal was passed averting market upheaval. 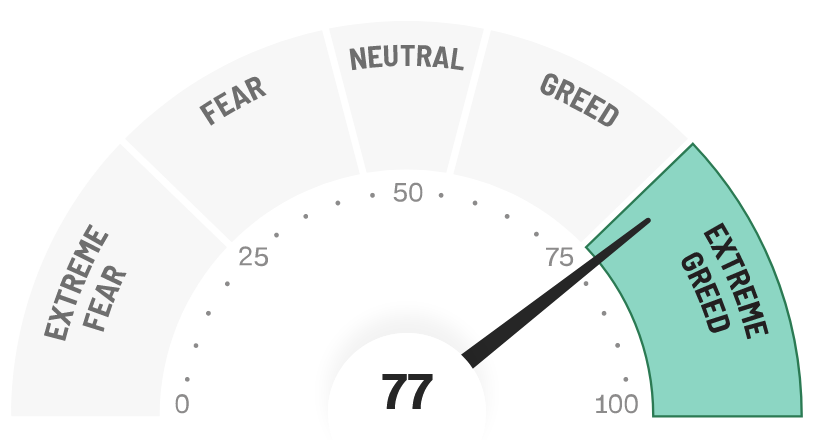
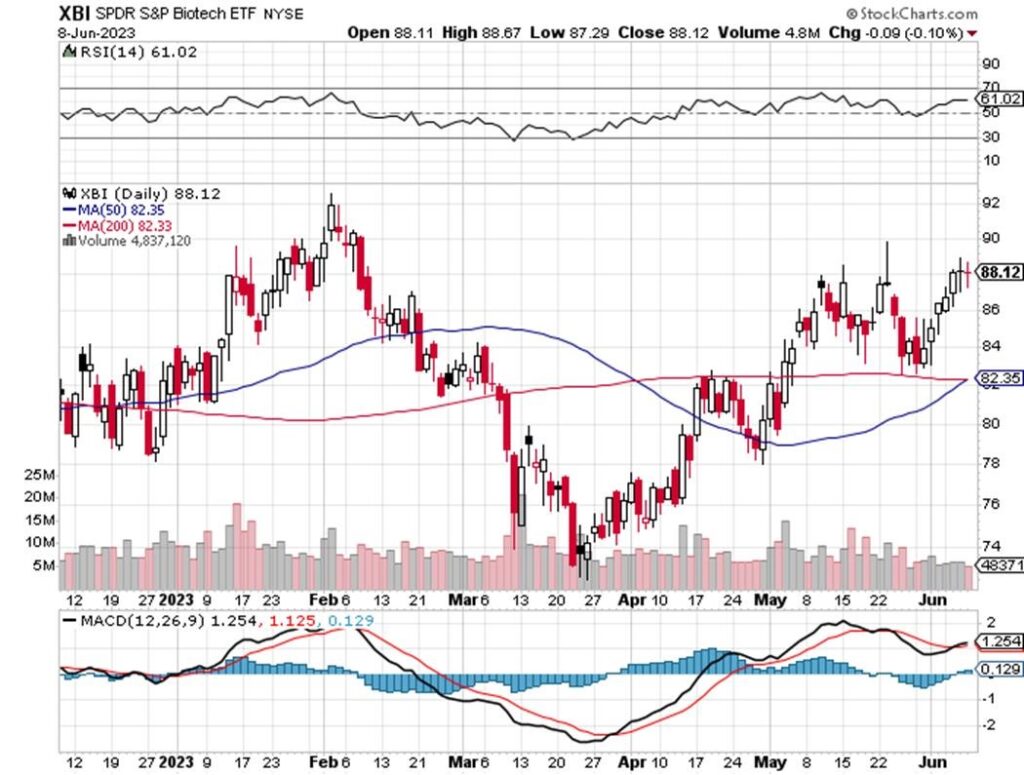
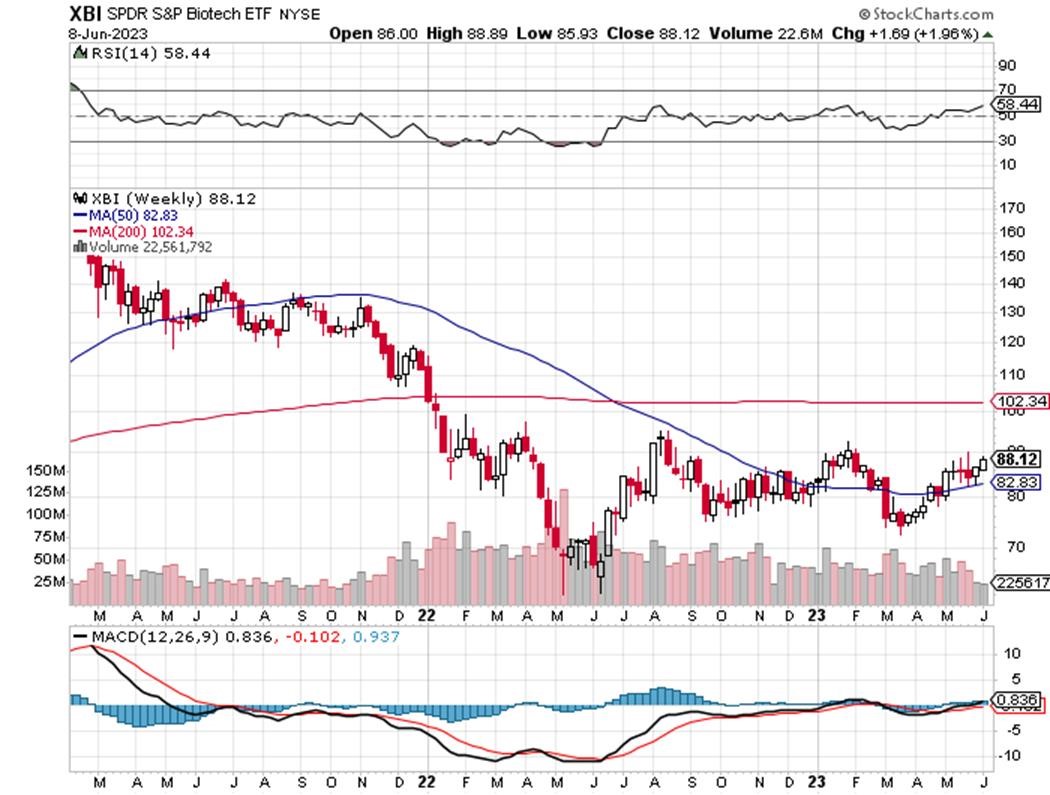
TECHNICALS – XBI Advances
The XBI Daily is up to 88.12 after closing at 83.55 two weeks ago. The 50 week MA is up nicely to 82 from 80 in the last Issue. The 200 week MA at 82 is flat for not just the last two weeks abut also for the last month. The RSI is up sharply to 61 from 49.
The XBI Weekly MA is down slightly to 82 after closing at 83 two weeks ago. The 200 week MA gap at 102 is slowly but surely being closed with the recent strength up from 88. The RSI is at 58 and definitely going in the right direction after closing at 52 two weeks ago.
MTSL Events Due Near-Term:
- European Hematology Association, June 8-11, INCY – 13 Abstracts featuring both late and early stage drug candidates for blood cancers
- American Diabetes Association, June 23-26, ESPR – Late Breaking Poster Session (printed poster), LDL Cholesterol Reduction and Cardiovascular Outcomes in High-Risk Primary Prevention Patients, June 24, 2023, 11:30 AM – 12:30 PM PT
- ePoster Theater (oral presentation), LDL Cholesterol Reduction and Cardiovascular Outcomes in High-Risk Primary Prevention Patients, June 24, 2023 5:40 PM – 5:50 PM PT, Presenter: Steven Nissen, MD, Chief Academic Officer of the Heart, Vascular & Thoracic Institute at Cleveland Clinic for the CLEAR Outcomes Investigators
- Q2 MDGL – File NDA application with FDA, Data at EASL June 21-24
- Q2 VKTX – Phase IIb NASH Data for VK-2809 (MRI-PDFF endpoint), Data at EASL June 21- 24
- CLDX – June 9-11 at EAACI 2023 barzo cholinergic urticaria data, barzo longer term CSU follow up, QOL data
Clinical Trials Watch
Company Updates
TCRT — TCRT Presents TCR Library Data at ASCO
TCRT recently presented updated data from the first three patients treated in its ongoing TCR-T Library Phase I/II trial at the 2023 ASCO. The poster highlighted the early clinical and translational data on the first three patients with refractory solid tumors expressing KRAS or TP53 mutations who received Sleeping Beauty TCR-T cells at one of two dose levels, DL1 (0.9 x 1010 TCR-T cells) and DL2 (6.4 x 1010 TCR-T cells and 5.8 x 1010 TCR-T cells). Manufactured TCR-T cells exhibited greater than 90% TCR positivity, viability and purity, underscoring the ability of the Company’s non-viral, universal manufacturing process to create TCR-T cells in multiple indications with different TCRs. Importantly, the TCR-T cells had a manageable safety profile and showed signs of efficacy in patients with mutant KRAS, EGFR and TP53-expressing solid tumors, including non-small cell lung (NSCLC), colorectal, endometrial, pancreatic, ovarian and bile duct cancers.
The data showed that in the overall best responses, Patient 1 (NSCLC) had a partial response with 6 month progression-free survival, Patient 2 (colorectal cancer) had stable disease and Patient 3 had progressive disease (pancreatic cancer). The 3 patients had cytokine release syndrome grades 2, 3 and 1, respectively, that were self-limiting or resolved with standard management and anti-IL-6 antibody (Patient 2). TCR-T cell persistence was observed in all patients at last follow-up (6 months for Patient 1). The trial may proceed to dose level 3 as long as safety continues to be manageable.
One of the keys to TCR-T therapies is understanding how the tumor microenvironment (TME) plays a role with this technology when delivering activated lymphocytes that have been modified or activated and expanded NK cells. Going forward it is important for clinical data to demonstrate that these cells really get into the tumor and have a treatment effect. Alaunos expects to provide an interim data update on multiple new patients in the third quarter of 2023 and anticipates establishing a recommended Phase II dose by year-end 2023. In or view, proof of concept is close to being established and the upcoming Phase II trial has the potential to create enough data/patients to file for an expedited FDA approval.
ALKS — ALKS Wins Royalty Fight With Janssen
The arbitraitors have ruled in ALKS favor against their partner JNJ/Janssen and they have been awarded $194 million in back royalties. The courts ruled that Janssen may terminate the license agreements, but they certainly may not continue to sell Products (as defined in the license agreements) developed during the term of the license agreements without paying royalties pursuant to the terms of the respective agreements;
– Back royalties related to U.S. sales in 2022 of approximately $194 million (inclusive of interest through March 15, 2023) are due to Alkermes from Janssen under the two agreements;
– A separate Know-How Royalty (as defined in the applicable license agreement) term applies for each of INVEGA SUSTENNA®, INVEGA TRINZA® and INVEGA HAFYERA®, as follows:
- The term for INVEGA SUSTENNA ends on 20, 2024;
- The term for INVEGA TRINZA ends in the second quarter of 2030 (but no later than May 2030when the applicable license agreement expires); and
- The term for INVEGA HAFYERA ends in May 2030(when the applicable license agreement expires); and
– Royalties for CABENUVA® in the U.S. are owed until Dec. 31, 2036.
The Janssen power play against ALKS has been a head scratcher for us from the beginning as how do you develop a series of drugs together, pay royalties for years, and then out of the blue claim the agreement is null and void. Bigger picture, the dispute may have helped ALKS as their focus has tightened and they are splitting the neruo from the oncology to make two separate entities.
At ASCO, ALKS presented two Trial-in-progress posters from the actively recruiting Phase II ARTISTRY-6 clinical trial and Phase III ARTISTRY-7 clinical trial. ARTISTRY-6 is evaluating nemvaleukin as a monotherapy in patients with advanced cutaneous melanoma or advanced mucosal melanoma. ARTISTRY-7 is evaluating nemvaleukin as a monotherapy and in combination with pembrolizumab in comparison to investigator’s choice chemotherapy in patients with platinum-resistant epithelial ovarian, fallopian tube or primary peritoneal cancer. We remain skeptical of ALKS’ IL-2 drug development candidate after witnessing NKTR’s problems, particularly the monotherapy, ARTISTRY-6. That being said ARTISTRY-7 has a small shot on goal being a combo therapy.
ESPR — ESPR Files sNDA With FDA For Cardio Benefit Based on CLEAR Positive Outcomes Trial, CLEAR Data at ADA
ESPR recently announced that it has submitted Supplemental New Drug Applications (sNDA) to the FDA for NEXLETOL and NEXLIZET in order to add the use of both NEXLETOL and NEXLIZET for cardiovascular risk reduction and also seeks to remove the statin limitation in the LDL-C indication.
The sNDA submissions are based on Cholesterol Lowering via Bempedoic acid, an ACL-Inhibiting Regimen (CLEAR) Outcomes trial which showed NEXLETOL demonstrated significant cardiovascular risk reduction across a range of primary and second endpoints, including a 27% risk reduction of non-fatal myocardial infarction, a 23% risk reduction of the composite of fatal and non-fatal myocardial infarction, a 19% risk reduction of coronary revascularization, a 15% risk reduction of the MACE-3 composite, and a 13% risk reduction of the MACE-4 composite.
Following the FDA’s review of the submission, it will notify the Company if the applications are deemed complete for review by mid-August 2023. The Company anticipates FDA approval of the sNDAs in the first half of 2024. These timeline are important as the significantly broader label that includes the CLEAR cardio outcomes benefit will allow payors to add the drugs to their formularies. The expanded label will also make the drugs much easier to sell which will dovetail perfectly with more insurers covering the drugs with expanded cardio outcomes benefit on the label.
ESPR Will Present CLEAR Data at the American Diabetes Association, June 23-26, 2023
Session Type: Late Breaking Poster Session (printed poster)
Date/Time: June 24, 2023, 11:30 AM – 12:30 PM PT
Abstract Title: LDL Cholesterol Reduction and Cardiovascular Outcomes in High-Risk Primary Prevention Patients
Session Type: ePoster Theater (oral presentation)
Date/Time: June 24, 2023 5:40 PM – 5:50 PM PT
Session Title: New Approaches and Renewing the Old – Late Breaking Abstracts
Presentation Title: LDL Cholesterol Reduction and Cardiovascular Outcomes in High-Risk Primary Prevention Patients
Presenter: Steven Nissen, MD, Chief Academic Officer of the Heart, Vascular & Thoracic Institute at Cleveland Clinic for the CLEAR Outcomes Investigators
ESPR continues to leverage the CLEAR data for a much broader label that will include the very important cardio/death benefit. The ADA will also be important as it will both illustrate the broad utility of NEXLETOL in lowering both diabetes and cardio risk while reducing LDL. The CLEAR data will also help differentiate the drug from statins which actually negatively increase A1C in diabetics. In our view, the sNDA filing will also help with the lawsuit against Diachhi in Europe.
IONS — IONS Completes Enrollment in Phase III HAE Trial (Data 1H2024), Sells $500 Million 2028 Convertible Notes to Replace 2024 Notes
IONS recently announced positive clinical progress with donidalorsen, its late-stage investigational prophylactic therapy for hereditary angioedema (HAE). Topline two-year open-label extension (OLE) results continue to demonstrate consistent efficacy and safety, with an overall sustained mean reduction in HAE attack rates of 96% from baseline through two years across dosing groups. The company also announced that it has completed enrollment in the Phase 3 OASIS-HAE study, which is evaluating the safety and efficacy of donidalorsen in preventing angioedema attacks. Topline data from the study are expected in the first half of 2024. HAE is a rare and potentially fatal genetic disease characterized by severe and potentially fatal swelling of the arms, legs, face and throat.
IONS announced the pricing of $500 million principal amount of 1.75% Convertible Senior Notes due 2028 (the “notes”) in a private placement (the “offering”) to qualified institutional buyers pursuant to Rule 144A under the Securities Act of 1933, as amended (the “Securities Act”). Ionis also granted the initial purchasers of the notes an option to purchase, within the 13-day period beginning on, and including, the date on which the notes are first issued, up to an additional $75.0 million aggregate principal amount of notes from Ionis. The sale of the notes is expected to close on June 12, 2023, subject to customary closing conditions.
The company expects to use approximately $420.4 million of the net proceeds from the offering to repurchase $434.1 million in aggregate principal amount of its 0.125% Convertible Senior Notes due 2024. Ionis expects to use the remaining net proceeds from the offering for additional repurchases of the 2024 notes from time to time following the offering, including the repayment of any remaining 2024 notes at maturity, and for general corporate purposes.
The HAE program is making very good progress and has also been partially de-risked by the ongoing positive data from Phase II. HAE is a great opportunity for IONS to sell the drug on their own and the more than 20,000 patients in the U.S. and Europe also represents a nice size market opportunity for the company. In our view, the Phase III HAE data in the first half of 2024 has a very good chance to read out positive and will serve as a strong stock catalyst for IONS.
PGEN — PGEN Presents ‘2009 & ‘3005 Data at ASCO, Phase II Up Next for ‘2009/PD-L1 Combo
PGEN reported positive data at ASCO combining PRGN-2009 with checkpoint inhibition that demonstrated a favorable safety profile and resulted in a 30% ORR with prolonged duration of responses in patients with heavily pre-treated HPV-associated cancers, including those who were checkpoint blockade resistant. The monotherapy arm only showed a modest effectiveness while the combo’s 30% ORR is a win for ‘2009. The company announced on May 31 that they have started a Phase II trial combining PRGN-2009 with a checkpoint inhibitor to further investigate safety and efficacy in recurrent or metastatic cervical cancer. The Phase II randomized, open-label, two-arm, multicenter trial will evaluate the efficacy and safety of PRGN-2009 in combination with pembrolizumab versus pembrolizumab monotherapy in patients with recurrent or metastatic cervical cancer who are pembrolizumab resistant. This new trial is the second Phase II for PRGN-2009 AdenoVerse, adding to the ongoing Phase II in oropharyngeal squamous cell carcinoma.
PGEN also had early data for PRGN-3005 at ASCO that showed it was well-tolerated with no dose limiting toxicities, no CRS greater than Grade 2, and no neurotoxicity. ‘3005 demonstrated expansion and persistence when delivered via either intraperitoneal or intravenous infusion without lymphodepletion or via intravenous infusion after lymphodepletion demonstrating the effectiveness of mbIL15.
A single intravenous infusion following lymphodepletion decreased tumor burden in 67% of the heavily pretreated patients (median of 8 or more prior therapies) with 90% of individual target lesions showing stable disease or partial response. The best responder achieved stable disease for more than 18 months after failing 9 prior lines of treatment. This is interesting but it looks like ‘3005 needs to tested in patients with fewer prior lines of therapy for their disease. ‘3005 is currently being evaluated in the Phase 1b dose expansion study at Dose Level 3 via IV infusion with lymphodepletion and incorporating repeat dosing.
The Phase II combo trial for ‘2009 is an important step and we look forward to this data after seeing a 30% ORR in Phase I. The data for ’3005 is still too early to fully evaluate as they are continuing the dose expansion phase. Overall, a solid ASCO for PGEN with the Phase II combo trial start for ‘2009 and the 30% ORR showing real promise.
VXRT — VXRT Raises Cash, Doses Last Subject in Phase II Norovirus Bivalent Trial
VXRT has announced that the last subject has completed dosing in the Phase II clinical trial of its oral pill bivalent norovirus vaccine candidate. In addition, all patients have been challenged in its challenge study of its G.1.1 monovalent vaccine candidate. No vaccine related SAEs have been reported to date in either trial, consistent with the safety profile observed in all our norovirus trials. The Company also expects to report topline data from the ongoing Phase II challenge study (NCT05212168) of Vaxart’s G1.1 monovalent norovirus vaccine candidate during Q3 2023. In our view, the two Phase II norovirus trials have the potential to provide proof-of-concept for the program and the oral vaccine platform.
The company continues to expect to report topline data from the ongoing Phase II dose-ranging study (NCT05626803) of its bivalent norovirus vaccine candidate in mid-2023. The primary endpoints are safety and immunogenicity, with the objective of determining dose levels for Phase III development.
VXRT just completed an offering of 16 million shares of its common stock, for gross proceeds of approximately $15 million. In our view, the timing was poor and the company might have waited for the two Phase II trials that are due to report mid-2023/Q3. Positive data would have allowed the company to raise at higher levels and avoided the significant dilution below $1. Despite the poorly timed raise, we still have confidence in the Phase II trials to deliver positive data. In our view, the two Phase II norovirus trials have the potential to provide proof-of-concept for the program and the oral vaccine platform.
The Back Page
| Symbol | Company | Orig.Rec. | Current | Target | Recommendation |
|---|---|---|---|---|---|
| ACAD | Acadia | 33.79 | 25.09 | 45 | BUY under $28 |
| ALKS | Alkermes | 10.13 | 31.54 | 55 | BUY under $35 |
| BCYC | Bicycle | 43.92 | 25.09 | 75 | BUY under $50 |
| BMRN | BioMarin | 12.68 | 92.26 | 150 | BUY under $100 |
| CLDX | Celldex | 10.50 | 35.57 | 100 | BUY under $75 |
| ESPR | Esperion | 24.42 | 1.35 | 25 | BUY under $10 |
| INCY | Incyte | 5.88 | 61.51 | 108 | BUY under $85 |
| IONS | Ionis | 7.63 | 41.65 | 65 | BUY under $50 |
| MDGL | Madrigal | 17.00 | 269.70 | 400 | BUY under $300 |
| PCRX | Pacira | 15.78 | 37.01 | 100 | BUY under $80 |
| PGEN | Precigen | 34.42 | 1.26 | 12 | BUY under $5 |
| SGMO | Sangamo | 4.77 | 1.21 | 15 | BUY under $7 |
| TCRT | Alaunos | 8.00 | 0.46 | 5 | BUY under $2 |
| VKTX | Viking | 16.83 | 24.79 | 45 | BUY under $28 |
| VXRT | Vaxart | 8.00 | 0.89 | 15 | BUY under $5 |
*New recommendation.
THE MODEL PORTFOLIO*
| COMPANY | SHARES OWNED | TOTAL COST | TODAY’S VALUE |
|---|---|---|---|
| Long Positions | |||
| Acadia | 4,750 | 156,557 | 119,178 |
| Alaunos | 26,125 | 166,100 | 12,018 |
| Alkermes | 3,800 | 88,690 | 119,852 |
| Bicycle | 2,400 | 105,408 | 60,216 |
| Celldex | 15,832 | 174,993 | 563,144 |
| Esperion | 3,316 | 105,316 | 4,477 |
| Incyte | 1,229 | 34,817 | 75,596 |
| Ionis | 3,087 | 49,123 | 128,574 |
| Madrigal | 3,127 | 69,980 | 843,352 |
| Pacira | 2,375 | 63,887 | 87,899 |
| Precigen | 9,690 | 76,510 | 12,209 |
| Sangamo | 19,456 | 253,596 | 23,542 |
| Vaxart | 29,687 | 250,000 | 26,421 |
| Viking | 12,000 | 201,960 | 297,480 |
| (06/08/23) | Equities: | $2,575,916 | |
| Cash: | $36,376 | ||
| PORTFOLIO VALUE: | $2,612,292 |
*The Model Portfolio is designed to reflect specific recommendations. We began the Model Portfolio on 12/23/83 with $100,000. On 4/13/84, we became fully invested. All profits are reinvested. Stocks recommended since then may be equally attractive, but may not be in the Model Portfolio. Transactions and positions are valued at closing prices. No dividends are created, and we don’t use margin. Interest income is credited only on large cash balances.
The Model Portfolio
BENCHMARKS
| NASDAQ | S&P 500 | MODEL | |
|---|---|---|---|
| Last 2 Weeks | 4.1% | 3.3% | 3.5% |
| 2023 YTD | 20.9% | 10.6% | 1.7% |
| Calendar Year 2022 | -33.1% | -19.4% | 12.7% |
| Calendar Year 2021 | 21.3% | 26.9% | -15.2% |
| Calendar Year 2020 | 43.6% | 16.3% | 13.8% |
| Calendar Year 2019 | 35.2% | 28.8% | 10.7% |
| Calendar Year 2018 | 5.7% | 6.6% | 4.5% |
| Calendar Year 2017 | 29.3% | 19.9% | 65.6% |
| Calendar Year 2016 | 7.5% | 9.5% | -29.6% |
| Calendar Year 2015 | -0.1% | -0.1% | 25.1% |
| Calendar Year 2014 | 13.4% | 11.4% | 29.2% |
| Calendar Year 2013 | 38.3% | 29.6% | 103.4% |
BENCHMARKS
New Money Buys
BioInvest
Contact Info
Medical Technology Stock Letter
John McCamant, Editor
Jay Silverman, Editor
Jim McCamant, Editor-at-Large
BioInvest.com
579 Mangels Ave.
San Francisco, CA 94127
510-843-1857
Send us an email
Download a PDF of MTSL Issue #1003
©Piedmont Venture Group (2023). Address: 579 Mangels Ave., San Francisco, CA 94127. Telephone: (510) 843-1857. Fax: (510) 843-0901. BioInvest.com. Email: admin@bioinvest.com. Published 24 times a year. Email subscription rates: 1 year – $399; 2 years – $678; 3 years – $898. You may cancel within 48 hours for a full refund. The information and opinions contained herein have been compiled or arrived at from sources believed to be reliable but no representations or warranty, express or implied, is made as to the accuracy or completeness. In no way shall this newsletter be construed as an offer to sell or solicitation of an offer to buy any securities. The publisher and its associates, directors or employees may have positions in, and may from time to time make purchases or sales of, securities mentioned herein. We cannot guarantee and you should not assume that future recommendations will equal the performance of past recommendations or be profitable.