September 2, 2019
BIOINVEST NEWS: Medicines Company (MDCO)
The Medicines Company (MDCO) – Inclisiran Indeed! Following up on the positive top-line results of the ORION 11 trial (released 8/26), the actual data presented at the ESC Congress 2019 were uniformly favorable for both efficacy and safety. In addition, despite the relatively smaller study and not powered for outcomes (n=1607), there was a strong reduction in Cardio Vascular Outcomes (CVO) in the Inclisiran treated arm versus placebo. Together, Inclisiran hit and, we believe, exceeded the very high bar that consensus were looking for. As one of the most widely watched studies in the biopharmaceutical industry, in our view, the data not only supports MDCO value’s proposition but may also might shift the recent negative investor perception of biotech stocks overall, just in time for the busy Fall season. REITERATE BUY
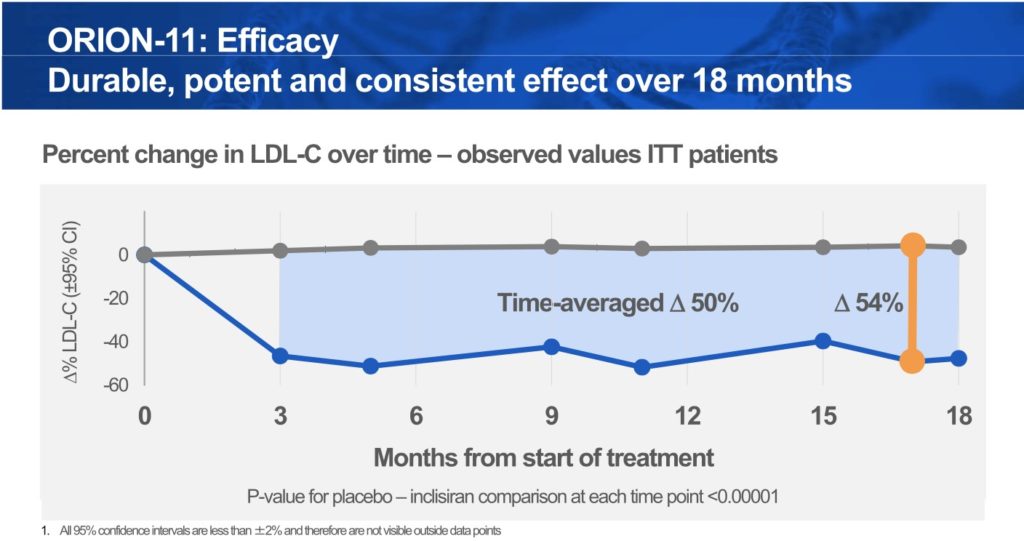
Efficacy – Persistent 50% LDL Reduction Over 4 Doses
The placebo-adjusted LDL-C reduction from baseline was 54% at Day 510 (-49% I vs. +4% P), achieving the 50%+ lowering that both physicians, payers and investors demand. The co-primary endpoint of time-averaged change in LDL-C from baseline at day 90 to day 540 was 50% (-48% I vs. +3% P)
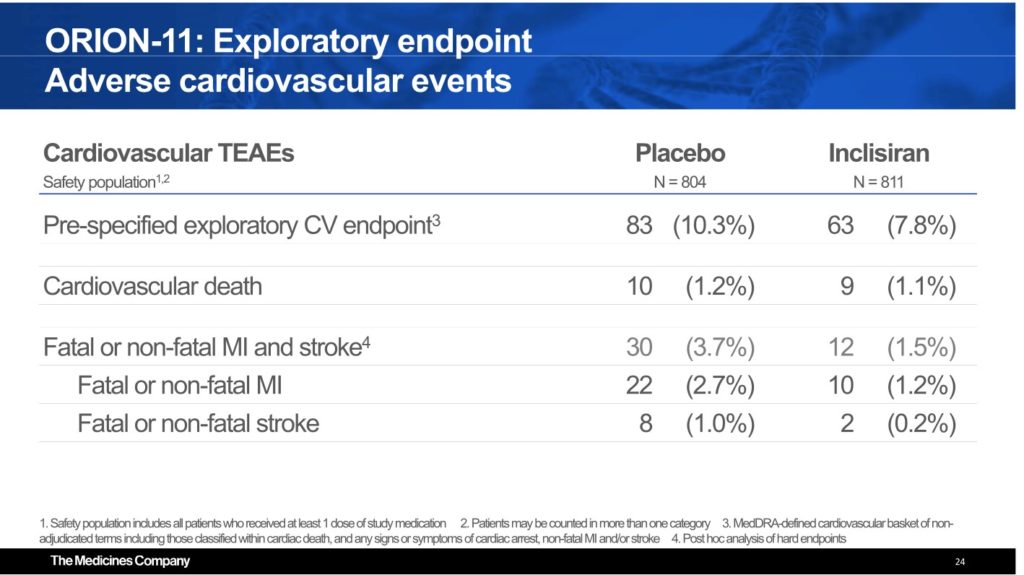
Strong Trend Support Outcomes Reduction
Although the trial was not powered to show any significant reduction in cardiovascular outcomes, Inclisiran appears to have reduced patient outcomes. The rate of fatal/non-fatal myocardial infarctions was 1.2% for Inclisiran vs 2.7% placebo and fatal/non-fatal stroke occurred in 0.2% of Inclisiran treated patients vs 1.0% placebo. If anything, ORION 11 provides further positive evidence of the ongoing and upcoming CVOT trials.
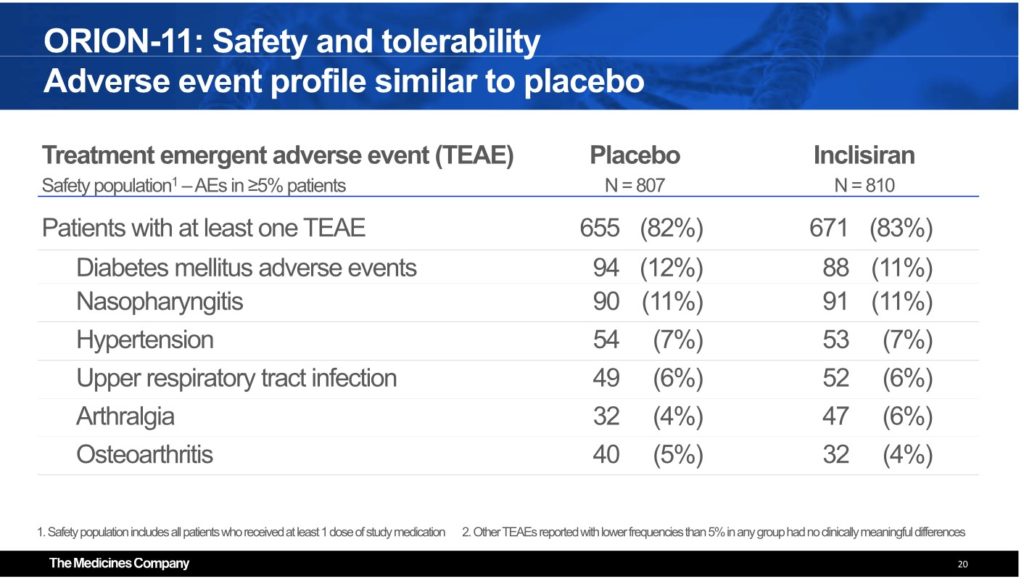
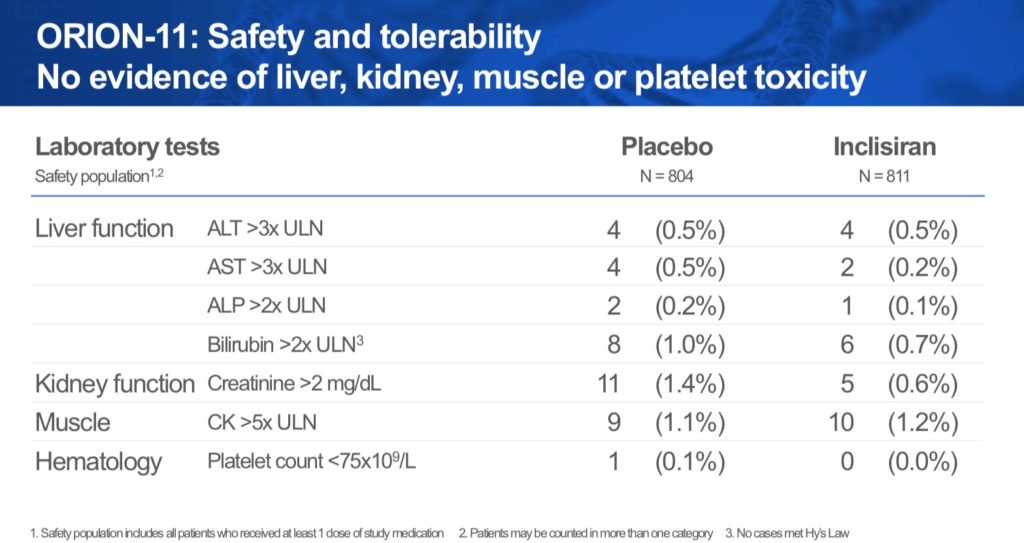
Pristine Safety Remains
With no margin for error, Inclisiran showed a remarkable safety profile that was quite similar to placebo. There were no treatment-related liver or renal abnormalities over the course of the study. Rates of ALT >3 ULN were 0.5% for both I and P, the rate of AST >3x ULN was 0.2% I and 0.5% for placebo, ALP > 2x ULN was 0.1% I and 0.2% P and bilirubin >2x ULN was 0.7% I compared with 1% for placebo. Creatinine >2 mg/dL was 0.6% for I vs 1.4% for P. Understandably, injection site reactions were higher for Inclisiran: 4.7% vs 0.5% placebo but all were either mild/moderate and none were persistent. Overall, TEAEs were balanced.
More Trials Ahead, NDA Filing – Takeout Potential Rises
ORION 11 was the first of three LDL lowering trials for Inclisiran to be released, with two more ORION trials due to report in September. The level of transparency of the entire ORION program is, we believe, an important indication of both the high end professionalism and quality of the MDCO management team. The Company will file an NDA in the US by year-end and in the EU by Q1:20.
Takeout Potential Rises
The ORION 11 trial completely de-risks the clinical profile of this unique and increasingly valuable asset that addresses the largest annual cause of death in the world, cardio vascular disease. Inclisiran provides patients and physicians with a safe, effective and extremely user friendly and affordable protocol to reduce LDL cholesterol compared with any other option available today. With a still modest market cap versus it’s commercial potential, in our view, MDCO is extremely undervalued.