February 25, 2024
BIOINVEST NEWS: Celldex (CLDX)
Celldex (CLDX) — Barzo Shines At AAAAI Meeting
On further inspection of the Phase II 12-week CSU data, barzolvolimab’s wide therapeutic window continues to support it Best-In-Class compound profile. The urticaria market is extremely underestimated and we expect barzo forecasts will rise significantly from here. BUY
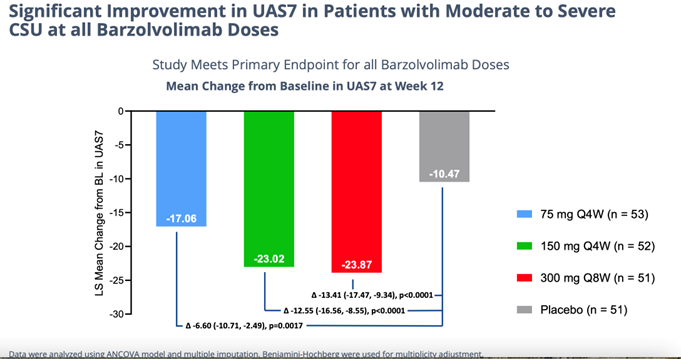
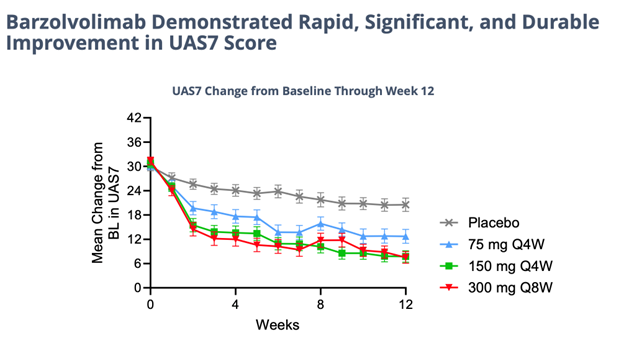
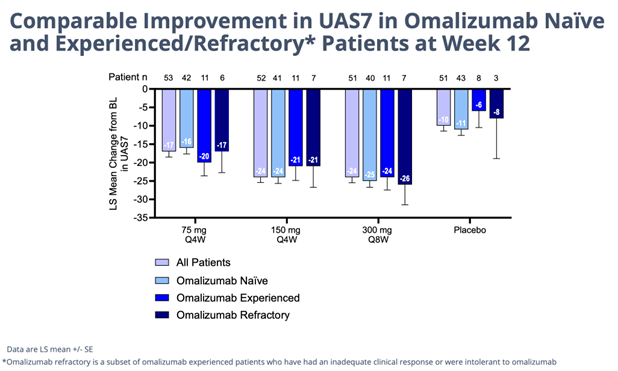
Unprecedented Efficacy… Details of the successful, 208-patient 12-week study study of barzo in CSU were presented in an oral Late-Breaker trial on Saturday at the AAAAI meeting. The drug’s novel advantageous mechanism of action is evident by the consistent, superior human clinical data delivered last November and shown again this weekend. In a highly severe patient group barzo showed a rapid onset of action (~2 weeks); durable, total disease control of CSU (but now in all forms of urticaria) – UAS7=0 was observed in 51% of patients in the 150mg Q4W and in 37.5% of patients in the 300 mg Q8W groups compared to 6.4% in the placebo group); and a more than acceptable safety profile. Moreover, the CLDX drug works just as good in Xolair (omalizumab) refractory, experienced and naive patients. Moreover, as in previous studies the responses with barzo deepen over time. Even though the data below is exceptional at just 12 weeks in and of itself, though not necessary to maintain its Best-in-Class profile, by 52 weeks the efficacy could possibly get even better.
…Supported By A Clean Safety Profile – One of the major investor (and bears/shorts) concerns coming into the meeting was the potential for neutropenia to be a limiting factor in barzo’s A/E profile. We have seen the efficacy presented above last November, but other than CLDX saying the safety was clean, until this weekend we had not yet seen the details. Of the 153 patients on Barzo in this study, just 2 patients discontinued due to neutropenia (Grade 3), with only 1 of those being confirmed by lab tests. However, Dr. Mauer quickly and effectively pointed out that one patient had pre-existing propensity for thrombocytopenia (not neutropenia) and the both of the patients briefly dropped below the pre-specified 1000 neutrophil count and had begun their recovery back above the threshold (one did it before and the other after the cutoff date). In other words, less than 1% of treated patients showed any sign of severe neutropenia and that resolves entirely over time and with drug discontinuation. According to both Drs. Maurer and Kaplan – barzo is a safe drug – and a much safer alternative than is currently being used to treat urticaria (e.g., steroids, cyclosporine, Xolair, the BTK inhibitors under development). In addition, in previous studies with the REGN/SNY blockbuster Dupixent, the rate of neutropenia in atopic dermatitis studies was over 4%. Both KOLs at the meeting easily dismissed neutropenia as a problem with barzo and reminded investors that every patient is pre-tested for these counts before beginning treatment anyway. In addition, Genentech’s Xolair – the only approved drug in the U.S for urticaria – carries a black box warning for anaphylaxis. Although there is always some risk in larger and longer term studies, at this stage of development the Barzo side effect profile looks very clean, easily refuting the bear/short call going into the meeting.
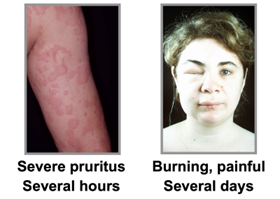
The Urticaria Market Is Much Bigger Than People Think – Both Mauer and Kaplan showed proof that urticaria and that chronic urticaria is a more devastating disease than most people are aware of, and with new treatments like Barzo this is about to change. Patients with CSU suffer constantly (with itch wheels, etc.), have an awful quality of life, and there is also a silence about the condition due to the physical stigma and lack of adequate treatments. Below we show some of examples the physical symptoms of urticaria. The vast number of CSU studies by Big Pharma and biotech firms suggest a huge market is approaching. Just wait until the advertising begins when new treatments like Barzo are approved.
While some older U.S. numbers have suggested a prevalence of 0.1-0.2% of the population, Mauer pointed out a more recent and vigorous epidemiology study of CSU done in China last year suggest a 2.6% prevalence and that the disease is even more severe in Asian and Latin American populations than in the US/EU.
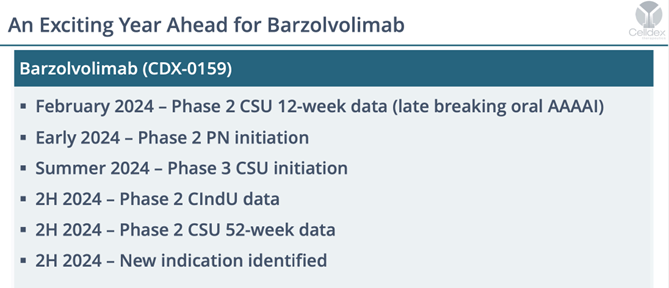
What’s Next for Barzolvilumab? A Whole Lot – The remainder of 2024 will include some major clinical catalysts including the start of Phase III studies that will support a BLA filing (summertime). Also, very soon we will get the start of Phase II prurigo nodular (PN) trials – another underestimated illness that Barzo has shown incredible (albeit small study) efficacy. In the second half, the Phase II CindU (cold induced urticaria) will come due and the all important 52-week data from this CSU trial will be out in H2 as well (likely by the November meetings).
Last But Not Least Is The 50->100 Conditions That Mast Cells Play A Key Role In – For a while now, we have pounded the table that Barzo will be a blockbuster drug. The AAAAI meeting further supports our hyphothesis. CLDX will announce a fifth clinical indication sometime in the second half of this year. but there are so many more. Dr. Kaplan, for example, yesterday mentioned that mast cells are over activated in synovial fluids of rheumatoid arthritis patients. Last week, we put out a note about food allergies. See our MTSL initiation report from August 2020. This is just the beginning.