January 27, 2023
BIOINVEST NEWS: Celldex (CLDX)
Celldex (CLDX) — Barzo Follow Up at AAAAI Is Exceptional
The full Phase 1b trial of barzolimab in chronic spontaneous urticaria (CSU) was presented yesterday and continues to support its potential as the best-in-class treatment for urticaria. On the conference call, Dr. Maeur said that as many as 30 – 75 mast cell diseases will respond to barzo – blockbuster status will be on the way. In our view, barzo has the potential to achieve Humira status, a $20 billion dollar drug that is approved for numerous autoimmune diseases. BUY
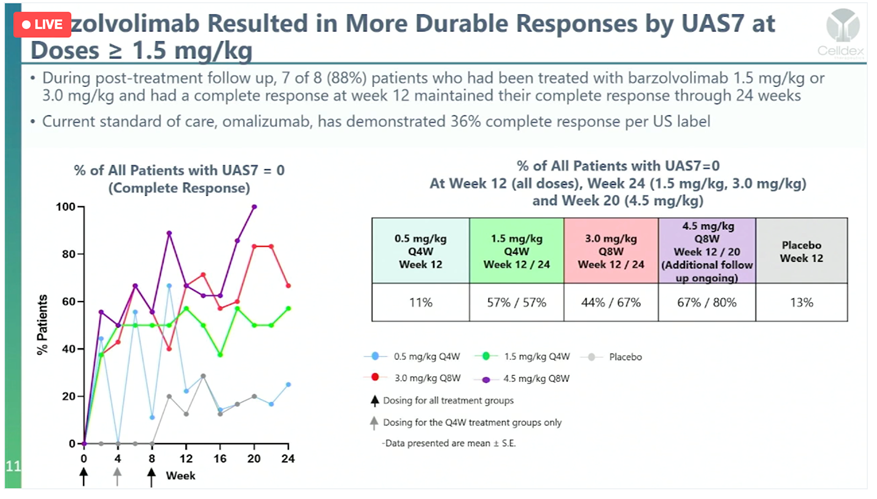
Greater Complete Responses At 24 Weeks (vs. 12 Weeks prior) AND At Higher Doses, Too
The data from the barzo follow up showed increased complete responses (CR) out to six months from the three months seen back in October 2021. To recall, last year data was available for the 1.5mg/kg dose group (two infusions four weeks apart) and at 3.0 mg/kg (only 1 infusion at that time). The new data includes the 3.0mg/kg and 4.5mg/kg full dose (2 infusions 8 weeks apart). At the two higher doses, the Complete Responses in the 3 mg/kg were strong and went from 44% at 12 weeks to 67% at 24 weeks; the 4.5 mg/kg were even more impressive – going from 67% at 12 weeks to 80% at 24 weeks. Unprecedented durable responses, and a major improvement from the strong three months of follow up since last year.
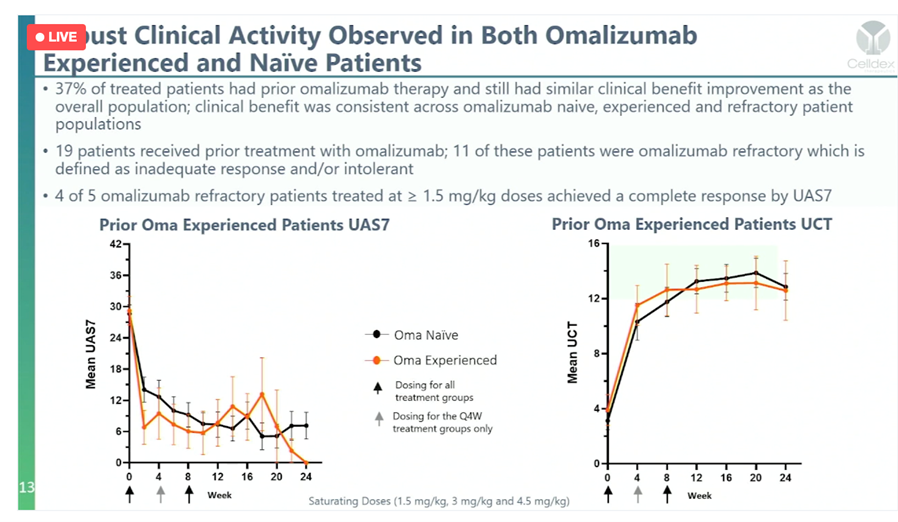
Works In OMA Resistance
Of the 5 patients in the study that failed Xolair treated with barzo, 4 of the 5 resulted in CRs. The results are spectacular whether or not the patient was naive to Xolair or Xolair refractory. Dr. Mauer said it best on the conference call- “barzo works where Xolair doesn’t.”
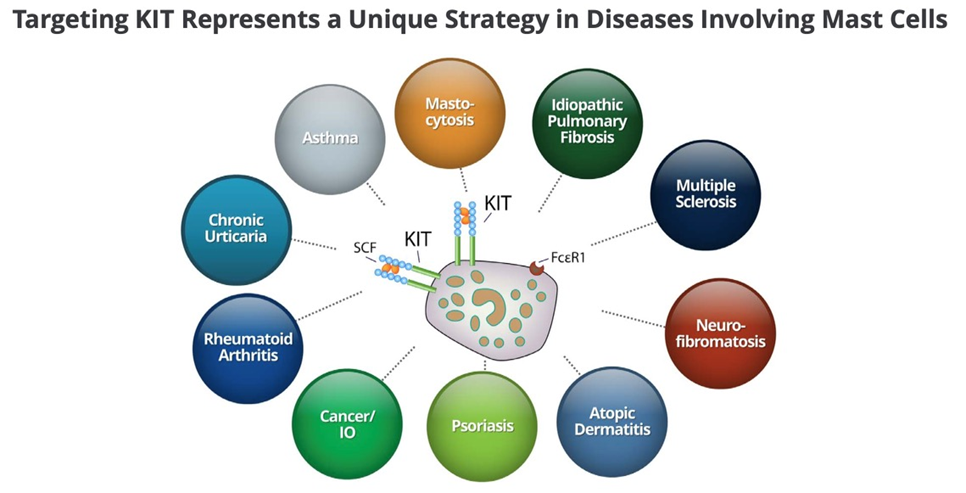
Dr. Mauer – “30-75 Mast Cell Diseases Will Probably Respond To Barzo”
CLDX has delivered excellent barzo data that is showing a strong treatment benefit and also very importantly very good safety. In our view, barzo is the best-in-class mast cell inhibitor in clinical development. In addition, barzo has the potential to work in 30-75 different mast cell diseases and achieve Humira status, a $20 billion dollar drug that is approved for numerous autoimmune diseases.