June 12, 2014
ASCO 2014 – Immune Oncology Rises to the Top – As many expected, immune oncology (I-O) was all the rage at the recently concluded American Society of Clinical Oncology (ASCO) meeting. While it is widely acknowledged that before ASCO, Bristol was the leader in the space followed by Merck, and Roche. At the conference, however, AstraZeneca showcased its I-O pipeline as the competitive landscape changes rapidly while the exciting sector evolves. BMS’s lead drug exhibited further toxicity in both NSCLC and renal trials, with some very real adverse events in lung cancer.  Yervoy has been the primary culprit, however, BMS also reported some notable adverse events with its PD-1 blocker, nivolumab. Just before ASCO on May 6, MRK was granted priority review for its top drug PD-1 prospect, MK-3475, with an FDA approval deadline of October 28. It is expected to get the first mover position in a new wave of immune-oncology therapies that is expected to be a game changer in the oncology market. The initial indication is melanoma.  Despite its speed bumps, BMS is still a leader but it is becoming clear that they may not have the best-in-class I-O drug candidates, leaving them vulnerable to the next wave of competitive I-O compounds. The emergence of AZN’s strong I-O pipeline is, in our view, the primary driver for the recent Pfizer bid.  Pfizer has little presence in the I-O sector and AZN’s emerging pipeline would have transformed Pfizer into an I-O player overnight.
Immune Oncology Market Poised for Significant Growth – The I-O sector is primed to become a super blockbuster market. Most current Wall Street estimates for the I-O treatment space (~$10-15 billion) are only based on few cancer types (melanoma, NSCLC, and renal) and that is changing. The signal at ASCO is that I-O will impact the vast majority, if not virtually all cancer types in some shape or form with all the new tools emerging.  A great example is the bladder cancer data reported by Roche which showed a 50% response rate (including one complete response) in PD-L1+ patients with RG-7446 in pre-treated metastatic bladder cancer; a setting which typically has very limited treatment options. Adding combination treatments to both radiation and chemo is another example of why the I-O market opportunity is poised to grow significantly larger over the next few years.
Big Pharma Dominates I-O By Combining Biotech Assets – While Big Pharma dominates the I-O treatment landscape, most of the assets and the technological know-how came from the biotech industry which pioneered the development of monoclonal antibodies. BMS acquired much of its I-O technology from the Medarex acquisition, Roche came into I-O through its Genentech investment, and AZN’s I-O platform is riddled with MedImmune technology.  We believe that INCY and FPRX are the two MTSL Recommendations that will have the biggest impact in the I-O treatment landscape. INCY through their unique and proprietary oral IDO-1 drug candidate and FPRX through their second generation antibody discovery platform that has already been validated by a major BMS collaboration.
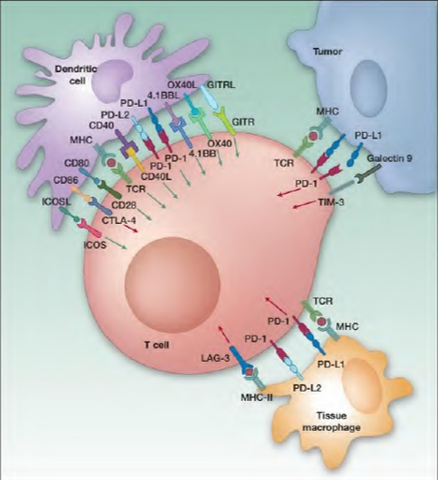
What is Immune Oncology? Immune oncology (I-O) is the newest and most exciting sector for new cancer drug development. Cancers grow and spread because tumor cells have developed ways to evade elimination by the immune system.  For example, cancer cells make proteins which apply the “brakes†to immune cells and prevent the immune cells from killing the tumor cells. One of the most exciting recent discoveries in cancer therapy has been the identification of ways to release these “brakes†and allow the immune cells to once again kill the tumor cells. This new approach has the potential of not only reducing tumor growth like traditional therapies, but potentially eliminating the cancer entirely in some patients. The approval of BMY’s Yervoy (ipilimumab which targets CTLA-4) to treat melanoma was a major breakthrough for cancer immune therapy – the drug had failed in multiple direct solid tumor trials before BMY harnessed its potential and garnered approval. Recently ipilimumab showed that some melanoma patients had lived as along as 10 years while on therapy. The PD-1/PD-L1 checkpoint pathway has recently emerged as the hottest target (and most competitive) for cancer immunotherapy in biotech with a slew of drug candidates in development (PD stands for “programmed cell deathâ€). While the aforementioned pathways have been grabbing all the headlines, IDO inhibitors are the next hot target in line behind the PD-1s. In our view, INCY’s INCB024360, an orally available small molecule that inhibits IDO (the others are injectable monoclonal antibodies), is rapidly emerging as the next major compound in I-O. In addition to being oral, ‘360 also may be a best in class drug which would make it a key part of combination therapy. Combo therapy is particularly important in I-O as this class of drugs appears to work better in combination and many experts expect three or more drugs could be used at once. This will also make safety very important for this class of drugs, because unknown and unpredictable toxicities can be a major problem when combining drugs. The NIH’s Dr. Louis Staudt referenced this exact point at the recent AACR meeting in April. There is much going on, but there is also much to learn.
AZN Unveils Broad I-O Portfolio at ASCO – AstraZeneca was one of the true surprises at ASCO as they have been busy building an I-O pipeline. The company will augment their current pipeline of drug candidates – CTLA4, PD1, PD-L1, with >9 new I-O molecules that will enter the clinic within next twelve months.
â– 1x new NME to take the T-cell breaks off
â– OX40 + 2xNMEs to put T-cell gas on
â– 1x NME in Tumor microenvironment
â– 4+x NMEs in Antigen presentation
Combination of tremelimumab (CTLA4) + PDL1 – The first dose ranging data was presented at AZN’s analyst meeting (this was too early to be shown in the full ASCO meeting and will be updated at ESMO).  Low toxicity burden means dose ranging is already much higher than BMS’ ipi+nivo combo and dose escalation continues. The ability to use a higher dose should bode well for ‘treme’s combo efficacy potential.  Better safety also bodes well for ‘treme in combo studies as toxicity will be a limiting factor for many drugs when used in 2’s or 3’s, which is the expectation with most I-O treatments.
MEDI4736 (PDL-1) – AZN will start an adjuvant lung study in 2015 with its PDL-1 under the theory that using I-O drugs earlier in the disease when the immune system is more intact will have a better affect. AZN made a specific point at their analyst meeting that bears watching going forward. They believe that PD-1 and PDL-1 may not be as interchangeable as is currently being assumed, and may actually have different effects on different cancers. This also explains why they have both PD’s currently in development.
MTSL Recommendations INCY & FPRX Have I-O Potential – I-O is clearly of high interest to investors and the standing room only crowd that eagerly greeted the presenter for INCY’s INCB24360 (IDO) poster is further illustration. In fact, the presenter was barely able to put it up for display due to crowding in the presentation area. For immunotherapy-naïve patients in the 25mg BID (n=8) and 50mg BID (n=4) cohorts, the objective response rate was 42% and the disease control rate was an impressive 75%. In immunotherapy-experienced patients (n=5) there were two patients with stable disease. Data from a 300mg subgroup had some intriguing follow-up data despite being stopped due to toxicity. Although all patients in the 300 mg twice-daily cohort were discontinued before responses could be fully evaluated, six of the seven patients were alive after one year, including three who have not received subsequent checkpoint inhibitor immunotherapy. In our view, this shows that the ‘360 may have a sustained treatment effect even after therapy has stopped. We remain impressed with the data for ‘360, albeit, from a small data sample.  For those who doubt IDO after the ASCO abstracts were made available, major votes of validation have occurred this year as INCY has already formed three IDO combination joint ventures with the leaders – MRK, BMY and AZN.
An IDO1 competitor, NewLink Genetics (NLNK) has its own compound in the clinic. Based on the data to date and stage of development, in our view, INCY is still the unquestioned leader in the IDO1 inhibitor space.  We anticipate multiple clinical updates over the next 12-24 months as the ‘360 combination studies mature. The fact that INCY has established three clinical research agreements to study ‘360 in combination with three of the four big players in I-O is further testament to the program’s strength. The collaborations are with anti-PD-1, MK-3475 (MRK), anti-PD-L1, MEDI 4736 (AZN), and with anti-PD-1, nivolumab (BMS). Given the prevalence of antibodies within the I-O drug development space, in our view, FPRX is extremely well positioned to be a major player in the future development of the sector.
New targets for cancer immunotherapy are needed to address those patients that do respond to or cannot tolerate agents currently in development. FPRX and their cutting edge second-generation antibody technology puts them in the proverbial “catbird seat†when it comes to identifying new targets and antibody drugs in this exciting and growing space.
Investment Conclusion – INCY, FPRX Well Positioned To Participate In The Next Blockbuster Oncology Market – As the I-O space evolves, MRK is currently in the lead, but by no means is it a zero sum game. BMS’ early lead appears less secure, with full data now being pushed to 2015. While being first to market is usually best, fast follow uppers can surprise as they use the information garnered by the leader to their advantage. This can be particularly important in clinical development and specific patient selection. AZN’s ‘treme is a very good example of a competitive late-comer as it is a second generation Yervoy and appears to have much less toxicity. The better toxicity profile should allow for increased dose vis-à -vis Yervoy leading to better efficacy. Additionally the better toxicity profile would make it a better combo drug than Yervoy as toxicity is paramount when combining multiple cancer drugs. While biomarkers are early in development for the I-O space, they could be important. For example, BMS’ biomarker may not be as accurate as MRK’s or ROG’s, which could lead to some confusion with their data.
In our view, INCY and FPRX are both well positioned to create value for their shareholders in the I-O sector. INCB24360 is a unique and potentially very active and safe drug candidate. The fact that the compound is already being developed with three of the biggest players in I-O (where are you Roche?) is a testament to both its uniqueness and potential. FPRX may have one of the most comprehensive tool kits to broadly address the I-O space. The company’s unique skill set allows it to both identify new targets and make the antibodies that address the new targets. The excellent BMS deal for preclinical I-O drug candidates is a major validation of FPRX’ unique antibody discovery/development platform.
